-
炎症是机体对外界刺激做出的保护性反应[1],对识别和破坏外源性病原体以维持内环境稳定至关重要[2]。机体发生炎症时,免疫细胞会产生异常水平的促炎因子,如NO、IL-6和TNF-α等,这些细胞因子在损伤部位募集与激活,会导致炎症的加剧[3]。研究表明,炎症与多种复杂疾病密切相关,如神经退行性疾病[4]、慢性风湿性关节炎[5]、肥胖症[6]、哮喘[7]、衰老[8]和癌症[9]等。
苦参碱(Matrine,Mat,图1)主要来源于豆科槐属植物[10],如苦参[11]和苦豆子[12]的根。苦参碱的分子结构由两个喹嗪环稠合而成,具有4个手性碳原子,绝对构型为5S、6S、7R和11R。苦参碱结构中有两个氮原子,16位氮原子为弱碱性酰胺氮,1位氮原子属于叔胺,三价都结合在环上,易于接受质子,因此赋予苦参碱较强的碱性,同时它也使苦参碱具有较大极性和良好水溶性。
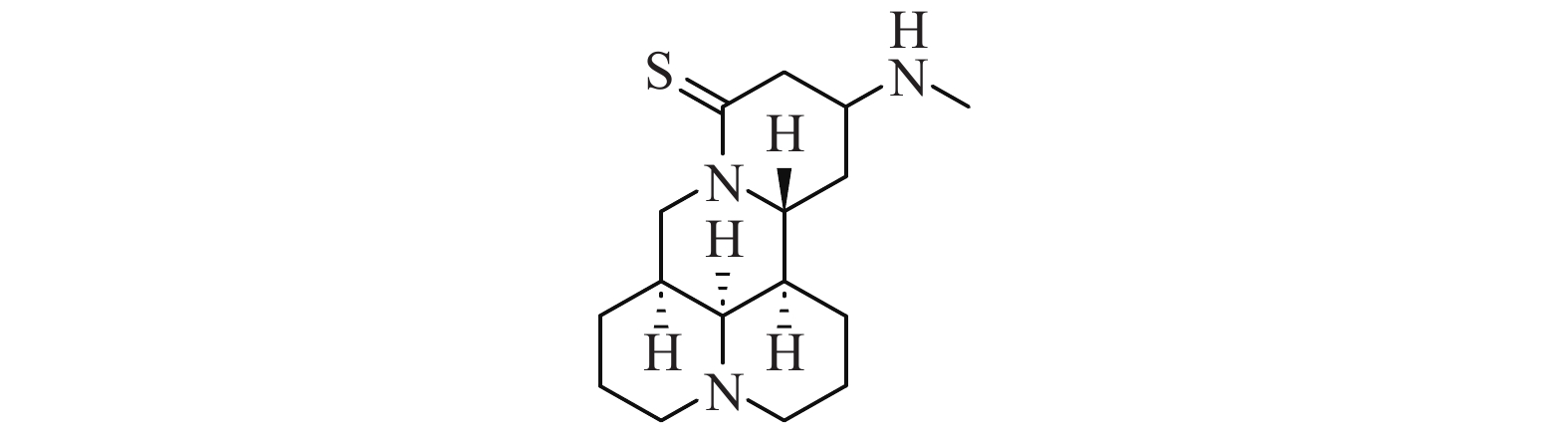
苦参碱是一种具有多种构象的异构体,包括椅式构象和船式构象,其中椅式构象为其优势构象[13]。苦参碱具有旋光性,有α、β、γ、δ 4种形态,这4种形态可以相互转化,其中最常用的是α-苦参碱[14]。Hu[15]以槐果碱为起始原料,经硫代和迈克尔加成反应,成功合成了13-甲氨基-15-硫代苦参碱(M19,图2),并对其进行体外抗炎活性研究,实验结果显示M19能显著抑制巨噬细胞中TNF-α的释放,具有良好的抗炎作用。
基于以上研究,本实验拟以苦参碱和M19为阳性对照,并借鉴与M19结构相似的苦参碱衍生物的抗炎构效关系,设计合成一类新型苦参碱衍生物。通过对这些衍生物进行系统的抗炎活性测试,旨在筛选出抗炎效果更优的候选化合物,为开发新型抗炎药物提供理论依据和实验基础。
-
AC-P核磁共振仪,美国Bruker Spectmspin公司;SW-CJ-2FD超净工作台,江苏苏净集团有限公司;CKX31临床级倒置显微镜,日本Olycmus公司;TDZ4台式低速离心机,湖南湘仪科学仪器有限公司;LSpectra Max M5E多功能酶标,仪美国Molecular Devices公司。
化学原料均为市售分析纯;RAW264.7细胞,中科院上海生物细胞研究所; CCK8试剂盒、一氧化氮检测试剂盒,上海碧云天生物技术有限公司;Mouse TNF-α ELISA Kit、Mouse IL-6 ELISA Kit,武汉伊莱瑞特生物科技股份有限公司。
-
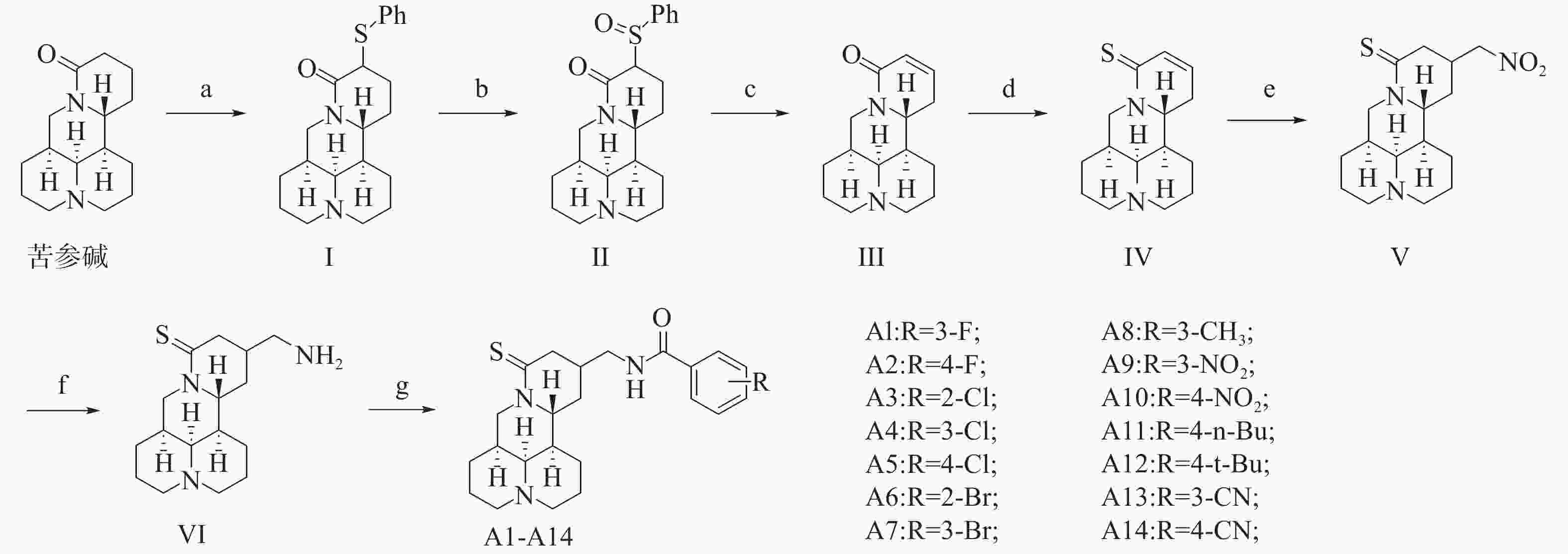
以苦参碱为起始原料,首先利用二异丙基氨基锂(LDA)脱去苦参碱14位α-H,再与二苯基二硫醚发生反应得到化合物 Ⅰ。接着,化合物 Ⅰ 在2-碘酰基苯甲酸的作用下氧化为化合物 Ⅱ,化合物 Ⅱ 在碳酸钾的作用下进一步得到化合物 Ⅲ,劳森试剂将化合物 Ⅲ 的羰基硫化为硫酮,得到化合物 Ⅳ,化合物 Ⅳ 与硝基甲烷和1,8-二氮杂双环[5.4.0]十一碳-7-烯发生迈克尔加成反应得到化合物 Ⅴ,锌粉将化合物 Ⅴ 的硝基还原为氨基,得到化合物 Ⅵ,化合物 Ⅵ 分别与取代苯甲酸反应,得到目标化合物,合成路线见图4。
-
取 250 ml干燥三颈瓶,氮气保护,加入70 ml四氢呋喃,降温至−78 ℃。加入二异丙胺(4.98 ml, 35.44 mmol),逐滴加入正丁基锂(14.77 ml, 35.44 mmol)的正己烷溶液(2.4 mol/L),−78 ℃下搅拌15 min。加入苦参碱(4.00 g, 16.11 mmol)的四氢呋喃溶液(20 ml),移出低温反应槽,室温搅拌1 h。加入二苯二硫醚(3.87 g, 17.72 mmol)的四氢呋喃溶液(10 ml),室温搅拌2 h。TLC(二氯甲烷∶甲醇∶氨水=20∶1∶1, v/v/v, 紫外及碘熏显色)显示反应完毕,停止反应。加入50 ml饱和碳酸钠溶液淬灭,用乙酸乙酯(3×200 ml)萃取,合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,减压蒸干,硅胶柱层析(二氯甲烷∶石油醚=1∶1→二氯甲烷∶乙酸乙酯=10∶1),得到 5.21 g 化合物 Ⅰ,黄色液体,收率90.8%。
-
取100 ml圆底烧瓶,加入50 ml水、浓盐酸(0.34 ml, 4.08 mmol)和化合物 Ⅰ (0.97 g, 2.72 mmol),搅拌至全溶,加入2-碘酰基苯甲酸(2.28 g, 8.16 mmol),控制外温50~70 ℃, 搅拌3 h。TLC(二氯甲烷∶甲醇∶氨水=20∶1∶1, v/v/v, 紫外及碘熏显色)显示反应完毕,停止反应。加入50 ml饱和碳酸氢钠溶液,用二氯甲烷(3×120 ml)萃取,合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,蒸干溶剂,得到化合物 Ⅱ 粗品,直接用于下一步反应。
-
向装有化合物 Ⅱ 粗品的100 ml圆底烧瓶中加入50 ml甲苯和碳酸钾(0.38 g, 2.72 mmol),置于110 ℃油浴中,回流1.5 h。TLC(二氯甲烷∶甲醇∶氨水=20∶1∶1, v/v/v, 紫外及碘熏显色),显示反应完毕,停止反应。向反应体系中加入50 ml 饱和碳酸钠溶液,用乙酸乙酯(3×120 ml)萃取,合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,减压蒸干,硅胶柱层析(二氯甲烷→二氯甲烷∶三乙胺=500∶1),得到0.61 g化合物 Ⅲ,白色粉末,收率 91.0%。m.p. 54.1~54.8 ℃;
-
取1 L三颈瓶,加入500 ml甲苯和化合物 Ⅲ(50.00 g, 203.11 mmol),置于120 ℃油浴中,加热回流,至完全溶清,开始回流时分批加入劳森试剂(45.18 g, 111.71 mmol),加入部分劳森试剂后,搅拌回流5 min,刮弃油状沉淀,TLC 监测反应液,2 h后TLC(二氯甲烷∶甲醇∶氨水=11∶1∶0.1, v/v/v, 紫外及碘熏显色)显示反应完毕,停止反应。趁热过滤,减压蒸干,硅胶柱层析(二氯甲烷→二氯甲烷∶甲醇=50∶1),得到30.30 g化合物 Ⅳ,淡黄色油状物,收率56.9%。
-
取500 ml圆底烧瓶,加入硝基甲烷(422.86 g, 6.93 mol)、化合物 Ⅳ(30.30 g, 115.46 mmol)和1,8-二氮杂双环[5.4.0]十一碳-7-烯(7.03 g, 46.18 mmol),室温搅拌过夜。TLC(二氯甲烷∶甲醇=10∶1, v/v, 紫外及碘熏显色)显示反应完毕,停止反应。蒸干反应液,加入100 ml饱和氯化铵水溶液,用二氯甲烷(3×200 ml)萃取,合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,减压蒸干,硅胶柱层析(二氯甲烷∶甲醇=50∶1),得到 34.01 g化合物 Ⅴ,黄色固体,收率 91.1%。m.p.139.4~141.3 ℃。
-
取500 ml圆底烧瓶,加入250 ml醋酸、化合物 Ⅴ(30.00 g, 92.83 mmol)和锌粉(24.28 g, 371.32 mol)室温搅拌过夜。TLC(二氯甲烷∶甲醇= 6∶1, v/v, 紫外及碘熏显色)显示反应完毕,停止反应。硅藻土过滤,蒸干反应液,加入100 ml饱和碳酸氢钠水溶液,用二氯甲烷(3×200 ml)萃取,合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,减压蒸干,硅胶柱层析(二氯甲烷∶甲醇∶三乙胺=70∶1∶0.1→二氯甲烷∶甲醇∶三乙胺=20∶1∶0.04),得到20.76 g化合物 Ⅵ,黄色油状物,收率76.3%。
-
取50 ml圆底烧瓶,氮气保护,加入10 ml 二氯甲烷、HOBt(165.79 mg, 1.23 mmol)、DIC(154.85 mg, 1.23 mmol)和3-氟苯甲酸(171.91 mg, 1.23 mmol),室温搅拌1 h,加入溶于5 ml 二氯甲烷的化合物 Ⅵ(120.03 mg, 0.41 mmol),室温搅拌过夜。TLC(二氯甲烷∶甲醇=10∶1, v/v, 紫外及碘熏显色)显示反应完毕,停止反应。减压蒸干,加入50 ml饱和碳酸氢钠水溶液,用二氯甲烷(3×100 ml)萃取,合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,减压蒸干,硅胶柱层析(二氯甲烷∶甲醇=50∶1),得到123.61 mg化合物 A1,白色油状物,收率72.7%。
参照合成目标化合物 A1 的合成方法,得到目标化合物 A2~A14。HR-MS和1H‐NMR、13C‐NMR数据见表1。
表 1 苦参碱衍生物HR-MS和1H-NMR、13C-NMR数据
化合物 收率(%) HR-MS(ESI)
[M+H]+
计算值/理论值核磁数据(δ ppm) VI 76.3 294.1998 /294.1984 1H NMR(600 MHz, DMSO-d6)δ 5.41(dd, J=13.0, 4.3 Hz, 1H), 4.23–4.18(m, 1H), 3.62(s, 1H), 3.44–3.37(m, 3H), 3.14(dd, J=19.3, 4.4 Hz, 1H), 3.11–3.07(m, 1H), 3.06–2.99(m, 2H), 2.79–2.73(m, 2H), 2.64(dd, J=18.1, 10.0 Hz, 1H), 2.13(t, J=15.3 Hz, 2H), 2.03(dd, J=42.2, 12.1 Hz, 3H), 1.77(dd, J=24.1, 9.9 Hz, 4H), 1.67(t, J=14.9 Hz, 4H), 1.55(dd, J=15.9, 11.5 Hz, 1H). 13C NMR(151 MHz, DMSO-d6)δ 196.37, 62.94, 54.75, 54.57, 54.49, 48.81, 45.70, 44.70, 42.05, 33.55, 26.35, 26.08, 24.88, 23.42, 18.63, 18.05. A1 72.7 416.2166 /416.2169 1H NMR(600 MHz, CDCl3)δ 8.28(t, J=5.8 Hz, 1H), 7.77(d, J=7.9 Hz, 1H), 7.70(dd, J=9.5, 2.0 Hz, 1H), 7.39(td, J=8.0, 5.7 Hz, 1H), 7.17(td, J=8.3, 2.5 Hz, 1H), 5.80(dd, J=13.7, 4.4 Hz, 1H), 4.79(dd, J=17.6, 9.3 Hz, 1H), 3.64(dt, J=13.6, 5.6 Hz, 1H), 3.57(d, J=11.7 Hz, 1H), 3.53(d, J=11.9 Hz, 1H), 3.46(t, J=13.6 Hz, 1H), 3.20(s, 1H), 3.13(dt, J=18.4, 2.8 Hz, 1H), 2.97(dd, J=18.4, 5.1 Hz, 1H), 2.84(ddd, J=13.8, 11.1, 6.1 Hz, 1H), 2.75(t, J=12.8 Hz, 2H), 2.57(dd, J=10.4, 5.1 Hz, 1H), 2.34–2.23(m, 2H), 2.18(t, J=14.7 Hz, 2H), 2.07(d, J=14.7 Hz, 1H), 1.94(d, J=14.1 Hz, 1H), 1.89–1.85(m, 1H), 1.81(td, J=9.4, 4.5 Hz, 2H), 1.72(d, J=15.2 Hz, 1H), 1.61(dd, J=22.6, 12.2 Hz, 2H). 13C NMR(151 MHz, CDCl3)δ 199.37, 166.76, 162.81(d, J=246.7 Hz), 136.19(d, J=6.8 Hz), 130.10(d, J=7.8 Hz), 122.91, 118.46(d, J=21.3 Hz), 114.69(d, J=23.0 Hz), 64.82, 56.57, 56.42, 52.97, 48.34, 45.38, 42.13, 41.51, 33.84, 28.31, 27.94, 25.61, 24.36, 18.98, 18.49. A2 66.4 416.2166 /416.2166 1H NMR(600 MHz, CDCl3)δ 8.23(t, J=5.7 Hz, 1H), 7.98(dd, J=8.8, 5.3 Hz, 2H), 7.09(t, J=8.6 Hz, 2H), 5.79(dd, J=13.7, 4.4 Hz, 1H), 4.76(dd, J=17.4, 9.3 Hz, 1H), 3.61(dt, J=13.5, 5.8 Hz, 1H), 3.57(d, J=11.9 Hz, 1H), 3.53(d, J=11.5 Hz, 1H), 3.45(t, J=13.6 Hz, 1H), 3.21(s, 1H), 3.13(d, J=2.5 Hz, 1H), 2.98(dd, J=18.4, 5.0 Hz, 1H), 2.90–2.84(m, 1H), 2.76(t, J=12.7 Hz, 2H), 2.55(dd, J=12.0, 7.1 Hz, 1H), 2.29–2.22(m, 2H), 2.17(dd, J=17.9, 14.2 Hz, 2H), 2.06(d, J=14.4 Hz, 1H), 1.96–1.91(m, 1H), 1.90–1.86(m, 1H), 1.84–1.79(m, 2H), 1.72(d, J=15.0 Hz, 1H), 1.65–1.59(m, 2H). 13C NMR(151 MHz, CDCl3)δ 199.31, 167.48, 164.93(d, J=251.9 Hz), 129.82(d, J=8.7 Hz), 115.50(d, J=21.8 Hz), 64.84, 56.57, 56.42, 53.02, 48.36, 45.37, 42.09, 41.59, 33.84, 28.30, 28.04, 25.58, 24.31, 18.98, 18.49. A3 78.3 432.1871 /432.1900 1H NMR(600 MHz, CDCl3)δ 7.57(dd, J=7.5, 1.7 Hz, 1H), 7.38(d, J=6.6 Hz, 1H), 7.32(dt, J=16.2, 7.4 Hz, 3H), 5.71(d, J=9.2 Hz, 1H), 4.66(d, J=11.4 Hz, 1H), 3.65–3.49(m, 4H), 3.20(s, 1H), 3.11(d, J=18.4 Hz, 1H), 3.07–3.01(m, 1H), 2.96(d, J=18.6 Hz, 1H), 2.69(t, J=12.8 Hz, 2H), 2.50(s, 1H), 2.24(d, J=11.6 Hz, 1H), 2.13(dd, J=34.0, 17.2 Hz, 4H), 1.91(d, J=13.9 Hz, 2H), 1.79(dd, J=21.9, 11.8 Hz, 3H), 1.71(d, J=15.3 Hz, 1H), 1.61(t, J=14.4 Hz, 1H). 13C NMR(151 MHz, CDCl3)δ 198.84, 167.51, 135.31, 131.24, 130.58, 130.15, 129.75, 127.14, 64.90, 56.37, 56.20, 53.81, 48.80, 45.59, 42.52, 41.40, 34.03, 28.15, 27.97, 25.72, 24.55, 19.00, 18.52. A4 49.9 432.1871 /432.1892 1H NMR(600 MHz, CDCl3)δ 8.25(s, 1H), 7.96(s, 1H), 7.85(d, J=7.7 Hz, 1H), 7.45(d, J=8.0 Hz, 1H), 7.36(t, J=7.9 Hz, 1H), 5.79(dd, J=13.8, 4.3 Hz, 1H), 4.79(dd, J=17.1, 9.0 Hz, 1H), 3.65–3.60(m, 1H), 3.56(dd, J=20.0, 11.8 Hz, 2H), 3.47(t, J=13.5 Hz, 1H), 3.21(s, 1H), 3.12(d, J=18.3 Hz, 1H), 2.99(dd, J=18.4, 4.9 Hz, 1H), 2.91–2.85(m, 1H), 2.75(t, J=12.5 Hz, 2H), 2.56(d, J=4.4 Hz, 1H), 2.27(t, J=11.4 Hz, 2H), 2.17(dd, J=13.3, 4.4 Hz, 2H), 2.06(d, J =14.4 Hz, 1H), 1.93(d, J=14.3 Hz, 1H), 1.88(d, J=11.0 Hz, 1H), 1.80(td, J=14.6, 4.5 Hz, 2H), 1.72(d, J=15.2 Hz, 1H), 1.64–1.59(m, 2H). 13C NMR(151 MHz, CDCl3)δ 199.31, 166.92, 135.60, 134.71, 131.60, 129.83, 127.87, 125.38, 64.86, 56.56, 56.43, 53.05, 48.39, 45.39, 42.04, 41.66, 33.85, 28.29, 27.93, 25.61, 24.36, 18.98, 18.50. A5 54.7 432.1871 /432.1898 1H NMR(600 MHz, CDCl3)δ 8.22(s, 1H), 7.90(d, J=8.5 Hz, 2H), 7.40(d, J=8.5 Hz, 2H), 5.80(dd, J=13.9, 4.4 Hz, 1H), 4.76(dd, J=17.7, 8.7 Hz, 1H), 3.61(dd, J=12.7, 7.2 Hz, 2H), 3.56(d, J=11.3 Hz, 1H), 3.46(t, J=13.6 Hz, 1H), 3.22(s, 1H), 3.12(d, J=18.6 Hz, 1H), 2.99(dd, J=18.4, 5.1 Hz, 1H), 2.93(ddd, J=13.8, 10.8, 6.3 Hz, 1H), 2.76(t, J=12.7 Hz, 2H), 2.56(d, J=4.7 Hz, 1H), 2.30–2.22(m, 2H), 2.19–2.13(m, 2H), 2.06(s, 1H), 1.95(d, J=13.6 Hz, 1H), 1.90(d, J=11.5 Hz, 1H), 1.81(dd, J=10.4, 5.5 Hz, 2H), 1.73(d, J=15.0 Hz, 1H), 1.67–1.61(m, 2H). 13C NMR(151 MHz, CDCl3)δ 199.37, 167.77, 138.15, 131.81, 128.91, 128.86, 65.01, 56.64, 56.50, 53.08, 48.39, 45.31, 42.04, 41.81, 33.85, 28.31, 27.95, 25.61, 24.35, 19.00, 18.52. A6 53.4 476.1366 /476.1388 1H NMR(600 MHz, CDCl3)δ 7.57(dd, J=8.0, 0.9 Hz, 1H), 7.47(dd, J=7.6, 1.6 Hz, 1H), 7.34(td, J=7.5, 1.0 Hz, 1H), 7.28–7.25(m, 2H), 5.71(dd, J=13.5, 4.5 Hz, 1H), 4.65–4.58(m, 1H), 3.59(dd, J=20.0, 8.6 Hz, 2H), 3.56–3.46(m, 2H), 3.22(s, 1H), 3.12(dd, J=18.4, 5.1 Hz, 1H), 3.08–3.04(m, 1H), 2.95(dd, J=18.3, 6.2 Hz, 1H), 2.71(dd, J=16.9, 8.8 Hz, 2H), 2.47(s, 1H), 2.25(d, J=8.4 Hz, 1H), 2.21–2.16(m, 1H), 2.11(q, J=12.2 Hz, 3H), 1.96–1.88(m, 2H), 1.83(ddd, J=13.7, 6.6, 3.0 Hz, 1H), 1.80–1.75(m, 2H), 1.72(d, J=15.3 Hz, 1H), 1.63(ddd, J=19.0, 10.2, 4.4 Hz, 1H). 13C NMR(151 MHz, CDCl3)δ 198.81, 168.76, 137.61, 133.32, 131.36, 129.30, 127.66, 119.22, 64.95, 56.40, 56.22, 53.85, 48.78, 45.61, 42.58, 41.31, 34.00, 28.18, 28.05, 25.66, 24.52, 18.99, 18.54. A7 44.6 476.1366 /476.1384 1H NMR(600 MHz, CDCl3)δ 8.20(s, 1H), 8.09(s, 1H), 7.87(d, J=7.6 Hz, 1H), 7.62(d, J=7.8 Hz, 1H), 7.30(t, J=7.9 Hz, 1H), 5.78(dd, J=13.4, 3.7 Hz, 1H), 4.77(s, 1H), 3.58(dd, J=19.5, 10.7 Hz, 3H), 3.48(t, J=13.5 Hz, 1H), 3.23(s, 1H), 3.09(d, J=18.0 Hz, 1H), 3.03–2.98(m, 1H), 2.96–2.90(m, 1H), 2.76(s, 2H), 2.54(s, 1H), 2.29–2.23(m, 2H), 2.15(d, J=13.0 Hz, 2H), 2.06(d, J=14.5 Hz, 1H), 1.91(dd, J=22.6, 12.7 Hz, 2H), 1.82(d, J=14.2 Hz, 2H), 1.73(d, J=14.7 Hz, 1H), 1.67–1.61(m, 2H). 13C NMR(151 MHz, CDCl3)δ 167.14, 135.61, 134.69, 130.78, 130.15, 125.86, 122.79, 64.97, 56.59, 56.47, 53.14, 48.48, 45.44, 41.94, 41.87, 33.86, 28.27, 27.91, 25.62, 24.38, 18.99, 18.51. A8 44.6 412.2417 /412.2434 1H NMR(600 MHz, CDCl3)δ 8.01(s, 1H), 7.73(s, 1H), 7.70(d, J=4.0 Hz, 1H), 7.31(d, J=4.6 Hz, 2H), 5.77(dd, J=13.8, 4.2 Hz, 1H), 4.74(s, 1H), 3.57(dd, J=17.0, 11.6 Hz, 3H), 3.49(t, J=13.5 Hz, 1H), 3.22(s, 1H), 3.06–2.94(m, 3H), 2.75(t, J=12.3 Hz, 2H), 2.53(s, 1H), 2.38(s, 3H), 2.28–2.19(m, 2H), 2.13(dd, J=12.9, 6.9 Hz, 2H), 2.05(d, J=14.7 Hz, 1H), 1.91(t, J=15.0 Hz, 2H), 1.84–1.77(m, 2H), 1.65(ddd, J=24.5, 22.5, 14.1 Hz, 3H). 13C NMR(151 MHz, CDCl3)δ 199.13, 169.11, 138.51, 133.39, 132.63, 128.51, 128.02, 124.39, 64.92, 56.53, 56.38, 53.35, 48.54, 45.48, 42.07, 41.79, 33.91, 28.17, 27.99, 25.61, 24.34, 21.26, 18.99, 18.50. A9 72.9 443.2111 /443.2139 1H NMR(600 MHz, CDCl3)δ 8.79(t, J=1.7 Hz, 1H), 8.62(t, J=5.8 Hz, 1H), 8.33–8.29(m, 2H), 7.60(t, J=8.0 Hz, 1H), 5.80(dd, J=13.7, 4.5 Hz, 1H), 4.76(dd, J=17.3, 9.7 Hz, 1H), 3.68–3.63(m, 1H), 3.60–3.53(m, 2H), 3.44(t, J=13.6 Hz, 1H), 3.24(s, 1H), 3.14(d, J=18.4 Hz, 1H), 2.98(dd, J=18.4, 5.1 Hz, 1H), 2.92(ddd, J=13.8, 10.9, 6.1 Hz, 1H), 2.81–2.75(m, 2H), 2.56(d, J=5.1 Hz, 1H), 2.33–2.23(m, 2H), 2.22–2.12(m, 2H), 2.08(d, J=14.1 Hz, 1H), 1.92(t, J=15.5 Hz, 2H), 1.85–1.80(m, 2H), 1.74(d, J=14.8 Hz, 1H), 1.67–1.61(m, 2H). 13C NMR(151 MHz, CDCl3)δ 199.38, 166.00, 148.32, 135.64, 133.36, 129.71, 126.01, 122.74, 64.84, 56.60, 56.47, 52.95, 48.32, 45.27, 42.08, 41.58, 33.80, 28.45, 27.99, 25.56, 24.31, 18.97, 18.48. A10 40.9 443.2111 /443.2130 1H NMR(600 MHz, CDCl3)δ 8.59(s, 1H), 8.28–8.25(m, 2H), 8.20–8.18(m, 2H), 5.83(dd, J=13.8, 4.4 Hz, 1H), 4.84(dd, J=16.8, 10.1 Hz, 1H), 3.70(dt, J=13.7, 5.7 Hz, 1H), 3.56(dd, J=26.9, 11.8 Hz, 2H), 3.46(t, J=13.5 Hz, 1H), 3.24–3.18(m, 2H), 2.97(dd, J=18.4, 5.0 Hz, 1H), 2.88(ddd, J=13.9, 11.5, 6.0 Hz, 1H), 2.78(s, 2H), 2.61(s, 1H), 2.31–2.26(m, 2H), 2.20(dd, J=22.6, 10.4 Hz, 2H), 2.09(d, J=14.2 Hz, 1H), 1.97(d, J=14.0 Hz, 1H), 1.85(ddd, J=16.1, 11.5, 5.0 Hz, 3H), 1.74(d, J=14.8 Hz, 1H), 1.67–1.58(m, 2H). 13C NMR(151 MHz, CDCl3)δ 199.43, 166.28, 149.73, 139.20, 128.77, 123.69, 64.94, 56.63, 56.54, 52.78, 48.26, 45.29, 42.27, 41.52, 33.77, 28.42, 27.92, 25.58, 24.32, 18.98, 18.52. A11 50.7 454.2887 /454.2937 1H NMR(600 MHz, CDCl3)δ 8.00(t, J=5.5 Hz, 1H), 7.84(d, J=8.2 Hz, 2H), 7.23(d, J=8.2 Hz, 2H), 5.77(dd, J=13.7, 4.4 Hz, 1H), 4.75(dd, J=18.1, 7.5 Hz, 1H), 3.60–3.54(m, 3H), 3.50(t, J=13.5 Hz, 1H), 3.21(s, 1H), 3.08–2.93(m, 3H), 2.75(t, J=12.4 Hz, 2H), 2.65–2.62(m, 2H), 2.54(d, J=4.4 Hz, 1H), 2.22(dd, J=30.6, 14.0 Hz, 2H), 2.18–2.10(m, 2H), 2.05(d, J=14.9 Hz, 1H), 1.91(t, J=16.3 Hz, 2H), 1.84–1.77(m, 2H), 1.73–1.64(m, 2H), 1.60(dq, J=15.3, 7.7 Hz, 3H), 1.38–1.30(m, 2H), 0.92(t, J=7.4 Hz, 3H). 13C NMR(151 MHz, CDCl3)δ 199.22, 168.75, 147.33, 130.75, 128.66, 127.36, 64.91, 56.52, 56.38, 53.32, 48.50, 45.46, 41.99, 41.78, 35.56, 33.89, 33.31, 28.17, 27.98, 25.61, 24.35, 22.33, 18.99, 18.51, 13.91. A12 58.8 454.2887 /454.2908 1H NMR(600 MHz, CDCl3)δ 8.02(t, J=5.5 Hz, 1H), 7.88(d, J=8.4 Hz, 2H), 7.45(d, J=8.4 Hz, 2H), 5.79(dd, J=13.7, 4.4 Hz, 1H), 4.76(dd, J=18.4, 7.7 Hz, 1H), 3.61–3.55(m, 3H), 3.50(t, J=13.4 Hz, 1H), 3.21(s, 1H), 3.08(d, J=18.8 Hz, 1H), 3.01(dd, J=18.4, 4.9 Hz, 1H), 2.93(ddd, J=13.8, 10.5, 6.1 Hz, 1H), 2.74(t, J=12.6 Hz, 2H), 2.56(dd, J=9.6, 4.8 Hz, 1H), 2.28–2.13(m, 4H), 2.05(d, J=14.7 Hz, 1H), 1.91(dd, J=26.8, 12.8 Hz, 2H), 1.80(ddd, J=14.0, 9.4, 4.3 Hz, 2H), 1.71(d, J=15.1 Hz, 1H), 1.66–1.57(m, 2H), 1.32(s, 9H). 13C NMR(151 MHz, CDCl3)δ 199.32, 168.54, 155.37, 130.56, 127.20, 125.56, 64.93, 56.53, 56.40, 53.25, 48.47, 45.45, 41.89, 41.85, 34.98, 33.88, 31.18, 28.18, 27.95, 25.63, 24.38, 18.99, 18.53. A13 69.5 423.2213 /423.2241 1H NMR(600 MHz, CDCl3)δ 8.54(t, J=5.8 Hz, 1H), 8.32(s, 1H), 8.24(d, J=8.0 Hz, 1H), 7.76(d, J=7.7 Hz, 1H), 7.55(t, J=7.8 Hz, 1H), 5.81(dd, J=13.7, 4.5 Hz, 1H), 4.79(dd, J=17.0, 9.8 Hz, 1H), 3.69–3.64(m, 1H), 3.55(dd, J=22.9, 11.4 Hz, 2H), 3.44(t, J=13.6 Hz, 1H), 3.23(s, 1H), 3.15(d, J=18.4 Hz, 1H), 2.98(dd, J=18.4, 5.0 Hz, 1H), 2.90–2.83(m, 1H), 2.78(s, 2H), 2.56(d, J=5.2 Hz, 1H), 2.29(t, J=13.5 Hz, 2H), 2.23–2.14(m, 2H), 2.08(d, J=14.5 Hz, 1H), 1.95(d, J=14.1 Hz, 1H), 1.90(d, J=11.0 Hz, 1H), 1.83(ddd, J=14.3, 9.7, 4.6 Hz, 2H), 1.75(d, J=15.1 Hz, 1H), 1.64(dt, J=19.4, 6.9 Hz, 2H). 13C NMR(151 MHz, CDCl3)δ 199.30, 166.10, 135.00, 134.72, 131.72, 131.45, 129.46, 118.20, 112.80, 64.85, 56.61, 56.48, 52.91, 48.31, 45.30, 42.17, 41.53, 33.80, 28.34, 28.01, 25.57, 24.29, 18.97, 18.50. A14 74.0 423.2213 /423.2242 1H NMR(600 MHz, CDCl3)δ 8.57(t, J=5.7 Hz, 1H), 8.12(d, J=8.4 Hz, 2H), 7.71(d, J=8.5 Hz, 2H), 5.82(dd, J=13.8, 4.4 Hz, 1H), 4.79(dd, J=17.0, 10.3 Hz, 1H), 3.71–3.66(m, 1H), 3.56(d, J=11.7 Hz, 1H), 3.51(d, J=11.9 Hz, 1H), 3.41(t, J=13.6 Hz, 1H), 3.24–3.15(m, 2H), 2.95(dd, J=18.4, 5.1 Hz, 1H), 2.82(ddd, J=25.2, 14.4, 8.7 Hz, 3H), 2.58(s, 1H), 2.29(dd, J=28.5, 14.4 Hz, 2H), 2.24–2.13(m, 2H), 2.07(d, J=14.5 Hz, 1H), 1.95(d, J=14.2 Hz, 1H), 1.90–1.79(m, 3H), 1.73(d, J=14.8 Hz, 1H), 1.66–1.56(m, 2H). 13C NMR(151 MHz, CDCl3)δ 199.34, 166.36, 137.67, 132.30, 128.20, 118.27, 114.95, 64.81, 56.61, 56.47, 52.74, 48.21, 45.27, 42.30, 41.38, 33.76, 28.37, 27.98, 25.55, 24.27, 18.96, 18.49. -
细胞生长状态良好且处于对数生长期时,调整细胞悬液浓度为2×105 个/ml,在96孔板的最外圈加入150 μl PBS,空白组加入100 μl DMEM(含10%FBS),对照组和实验组均加入100 μl细胞悬液(2×105 个/ml),将96孔板置于二氧化碳培养箱中,在37 ℃、5% CO2条件下培养14 h。培养结束后,吸弃空白组、对照组和实验组的培养基,空白组和对照组加入100 μl DMEM(含10%FBS),实验组每孔分别加入100 μl浓度为3.125、6.25、12.5、25和50 μmol/L的苦参碱衍生物溶液,将96孔板重新置于二氧化碳培养箱中培养24 h。吸弃各组培养基,每孔加入100 μl配制好的CCK8溶液(CCK8∶DMEM=1∶10),将96孔板放入二氧化碳培养箱中培养2 h。孵育结束,使用酶标仪在450 nm波长下检测各孔的吸光度,并计算细胞活力。每组实验设立4个复孔,并重复实验3次。
$$ \text{细胞活力}(\text{%})=\frac{\text{实验组吸光度}-\text{空白组吸光度}}{\text{对照组吸光度}-\text{空白组吸光度}}\times 100\text{%} $$ -
细胞生长状态良好且处于对数生长期时,调整细胞悬液浓度为2×105 个/ml,在96孔板的最外圈加入150 μl PBS,空白组、对照组和实验组均加入100 μl细胞悬液,将96孔板置于二氧化碳培养箱中,在37 ℃、5% CO2条件下培养14 h。吸弃空白组、模型组和实验组的培养基,空白组和模型加入100 μl DMEM(含10%FBS),实验组加入100 μl 12.5 μmol/L的苦参碱衍生物,在二氧化碳培养箱中培养4 h。模型组和实验组加入2 μl LPS(5 ng/μl),在二氧化碳培养箱中培养14 h。每孔吸出50 μl上清液,加到新96孔板中,加入50 μl Griess Reagent Ⅰ和50 μl Griess Reagent Ⅱ,使用酶标仪检测苦参碱衍生物在540 nm处的吸光度,计算NO含量。每组设立4个复孔,重复实验3次。
$$ \text{相对抑制浓度}(\text{%})=({1}-\frac{\text{实验组NO含量}}{\text{对照组NO含量}})\times 100\text{%} $$ -
细胞生长状态良好且处于对数生长期时,调整细胞悬液浓度为2×105 个/ml,在96孔板的最外圈加入150 μl PBS,空白组、对照组和实验组均加入100 μl细胞悬液,将96孔板置于二氧化碳培养箱中,在37 ℃、5% CO2条件下培养14 h。吸弃空白组、模型组和实验组的培养基,空白组和模型加入100 μl DMEM(含10% FBS),实验组加入100 μl 12.5 μmol/L的苦参碱衍生物,在二氧化碳培养箱中培养4 h。模型组和实验组加入1 μl LPS(2.5 ng/μl),在二氧化碳培养箱中培养14 h。收集上清液,−20 ℃保存,根据ELISA试剂盒说明书检测IL-6的含量。每组设立4个复孔,重复实验3次。
$$ \text{相对抑制浓度}(\text{%})=({1}-\frac{\text{实验组IL}-6\text{含量}}{\text{对照组IL}-6\text{含量}})\times 100\text{%} $$ -
细胞生长状态良好且处于对数生长期时,调整细胞悬液浓度为2×105 个/ml,在96孔板的最外圈加入150 μl PBS,空白组、对照组和实验组均加入100 μl细胞悬液,将96孔板置于二氧化碳培养箱中,在37 ℃、5% CO2条件下培养14 h。吸弃空白组、模型组和实验组的培养基,空白组和模型加入100 μl DMEM(含10%FBS),实验组加入100 μl 12.5 μmol/L的苦参碱衍生物,在二氧化碳培养箱中培养4 h。模型组和实验组加入2 μl LPS(5 ng/μl),在二氧化碳培养箱中培养14 h。收集上清液,−20 ℃保存,根据ELISA试剂盒说明书检测TNF-α的含量。每组设立4个复孔,重复实验3次。
$$ \text{相对抑制浓度}(\text{%})=({1}-\frac{\text{实验组TNF}-\alpha \text{含量}}{\text{对照组TNF}-\alpha \text{含量}})\times 100\text{%} $$ -
苦参碱衍生物对RAW264.7细胞的细胞毒性结果如表2所示。浓度为50 μmol/L时,M19的细胞存活率为52.87±0.41%,中间体 Ⅵ 的细胞存活率为95.19±0.59%。新型苦参碱衍生物的细胞存活率均高于M19,细胞毒性得到了显著改善。苦参碱衍生物浓度为12.5 μmol/L时,所有衍生物的细胞存活率均大于90%,且浓度适中,故选择12.5 μmol/L的浓度进行抗炎活性研究。
表 2 苦参碱及其衍生物对 RAW 264.7的细胞毒性(n=3)
化合物 细胞存活率(%) 3.12 μmol/L 6.25 μmol/L 12.5 μmol/L 25 μmol/L 50 μmol/L 苦参碱 99.42±0.19 97.24±0.42 96.29±0.59 94.24±0.28 89.13±0.06 M19 94.09±0.57 92.76±0.41 90.74±0.20 80.66±0.38 52.87±0.41 VI 98.10±1.04 97.25±0.78 96.73±0.61 96.29±0.92 95.19±0.59 A1 99.50±0.23 98.71±0.46 96.21±0.93 94.19±0.45 91.85±0.76 A2 99.60±0.21 98.81±0.54 96.71±0.51 93.78±1.18 90.77±0.81 A3 98.89±0.77 96.84±0.86 93.96±0.59 93.20±0.46 90.52±1.07 A4 99.84±0.16 98.69±0.38 97.59±0.92 95.59±1.19 94.35±0.81 A5 99.64±0.28 98.73±0.25 97.73±0.93 94.52±0.53 90.92±0.71 A6 98.75±0.86 95.81±0.61 92.74±0.28 88.37±1.23 83.62±0.52 A7 96.01±1.04 94.42±0.47 92.40±0.37 83.15±0.47 68.15±0.82 A8 95.93±0.75 94.49±0.96 92.97±1.18 83.94±1.21 76.86±0.72 A9 99.40±0.16 94.93±0.30 92.33±0.23 88.89±0.39 75.29±0.57 A10 99.20±0.27 96.31±0.57 94.89±0.29 86.78±1.22 80.82±1.23 A11 97.87±0.22 95.87±0.70 92.81±0.29 86.60±0.61 55.31±0.84 A12 97.88±0.44 94.68±0.59 91.92±0.46 83.68±0.39 76.95±0.74 A13 97.57±0.54 95.36±1.21 94.95±0.53 83.86±0.66 81.46±0.33 A14 95.59±0.70 94.76±0.64 92.90±0.57 82.33±0.73 78.85±0.66 -
化合物对NO释放的抑制作用越强,其NO抑制率越高,抗炎活性也越显著。由表3数据可知,苦参碱的NO抑制率为40.95±0.73%,而M19的NO抑制率为64.39±0.66%,化合物 Ⅵ 的NO抑制率为55.93±0.75%,这些结果表明,在苦参碱15位引入硫酮基团、13位引入氮原子能够显著增强抗炎活性。
表 3 苦参碱衍生物在12.5 μmol/L下对LPS诱导的RAW264.7细胞的NO释放的影响(n=3)
化合物 NO抑制率(%) 化合物 NO抑制率(%) 苦参碱 40.95±0.73 A7 57.33±0.53### M19 64.39±0.66### A8 55.14±0.34### VI 55.93±0.75### A9 58.92±0.10### A1 56.62±0.73### A10 69.40±0.28*** A2 57.13±0.25### A11 67.50±0.44*** A3 60.01±0.53### A12 72.70±0.58*** A4 61.27±0.24### A13 61.31±0.36### A5 63.61±0.38### A14 63.62±0.37### A6 67.22±0.99** **p<0.01, ***p<0.001 与M19相比; ###p<0.001 与苦参碱相比 进一步分析发现,所有新型苦参碱衍生物的NO抑制率(50.47±0.40~72.70±0.58%)均高于苦参碱。其中,化合物A6(67.22±0.99%)、A10(69.40±0.28%)、A11(67.50±0.44%)、A12(72.70±0.58%)的NO抑制率甚至超过了M19,尤其是化合物A12表现出最强的NO抑制活性。这一结果显示,通过合理的结构修饰,可以显著提升苦参碱衍生物的抗炎效果。
-
化合物对IL-6和TNF-α释放的抑制作用越强,其IL-6和TNF-α抑制率越高,抗炎活性也越强。由表4可知,所有参与测试的新型苦参碱衍生物在抑制IL-6和TNF-α释放方面均表现出优于M19的效果。其中,化合物A11(86.21±0.51%)和A12(86.64±0.33%)对IL-6的抑制率显著高于M19(59.77±0.39%);同时,化合物A12对TNF-α的抑制率(71.24±1.25%)也明显高于M19(49.57±0.92%)。
表 4 苦参碱衍生物在12.5 μmol/L下对LPS诱导的RAW264.7细胞的IL-6和TNF-α释放的影响(n=3)
化合物 IL-6 抑制率(%) TNF-α 抑制率(%) M19 59.77±0.39 49.57±0.92 A6 63.18±0.48*** 56.7±1.02*** A10 69.84±0.32*** 52.2±1.36* A11 86.21±0.51*** 55.46±0.61*** A12 86.64±0.33*** 71.24±1.25*** *p<0.05, **p<0.01, ***p<0.001与 M19相比 综合上述实验结果,化合物A12在抑制IL-6和TNF-α释放方面表现出最强的活性,显示出最优的抗炎效果,表明其具有进一步开发为高效抗炎药物的潜力。
-
苦参碱作为一种天然生物碱,具有显著的抗炎活性,但其细胞毒性和抗炎活性强度仍有优化空间。本研究通过对苦参碱进行结构修饰,设计并合成了一类新型苦参碱衍生物,并系统评价了其抗炎活性及细胞毒性。研究结果表明,新型苦参碱衍生物的细胞毒性均显著低于M19,表明在苦参碱 15 位引入硫酮基团, 13 位引入氨甲基或酰胺基能够有效降低其细胞毒性,为结构修饰提供了重要参考。
所有新型苦参碱衍生物的NO抑制率均高于苦参碱,部分衍生物甚至优于M19(64.39±0.66%),其中化合物A12(72.70±0.58%)表现出最优的NO抑制活性。进一步分析发现,新型苦参碱衍生物13位苯环上的取代基,无论是吸电子基还是给电子基,均能赋予衍生物良好的抗炎活性。抗炎活性强弱顺序总体表现为对位>间位>邻位(除溴原子外)。此外,当13位苯环上的取代基为烷基时,支链越多,抗炎活性越强。这一发现为后续的结构优化提供了重要指导。
综上所述,本研究通过合理的结构修饰,成功开发出具有低毒性和高效抗炎活性的苦参碱衍生物,尤其是化合物A12表现出显著的开发潜力。这一发现不仅验证了结构修饰的有效性,也为苦参碱衍生物的进一步研究与开发奠定了重要基础。未来研究可进一步探索其作用机制及体内药效,为开发新型抗炎药物奠定基础。
Design, synthesis, and in vitro anti-inflammatory activity of novel Matrine derivatives
-
摘要:
目的 中药苦参中含有的苦参类生物碱具有良好的抗炎活性,本研究通过对苦参碱进行结构修饰,探讨新型苦参碱衍生物的结构与抗炎活性之间的构效关系。 方法 以苦参碱为先导化合物,通过化学修饰得到14个新型苦参碱衍生物。以苦参碱和 M19 为阳性对照,采用细胞计数试剂盒-8(CCK-8)法检测苦参碱衍生物对RAW264.7细胞的细胞毒性,使用一氧化氮(NO)检测试剂盒检测苦参碱衍生物对脂多糖(LPS)诱导的RAW264.7细胞炎症模型NO生成的抑制作用,并采用酶联免疫吸附试验(ELISA)检测苦参碱衍生物对LPS诱导的RAW264.7细胞炎症模型中白细胞介素-6(IL-6)和肿瘤坏死因子-α(TNF-α)分泌的影响。 结果 与 M19 相比,所有新型苦参碱衍生物均表现出较低的细胞毒性;在NO抑制方面,所有衍生物的活性均优于苦参碱,部分衍生物活性甚至超过 M19 ,其中化合物 A12 显示出最优的NO抑制率;化合物 A11 和 A12 的IL-6抑制活性优于对照 M19 ;在TNF-α抑制方面,化合物 A12 同样表现出最优异的活性。 结论 化合物 A12 在抑制NO、IL-6和TNF-α释放方面表现出最强的活性,具有最佳的抗炎效果,为后续深入研究提供了重要的先导化合物。 Abstract:Objective The alkaloids contained in the Chinese herb Sophora flavescens have good anti-inflammatory activity. To investigate the structure-activity relationship between the novel Matrine and the anti-inflammatory activity by modifying the structure of Matrine . Methods Fourteen novel Matrine derivatives were obtained by chemical modification using Matrine as the lead compound with Matrine and M19 as positive controls. The cytotoxicity of Matrine derivatives against RAW264.7 cells was detected by the Cell Counting Kit 8 (CCK8) assay, and the relative amount of Nitric Oxide (NO) produced by Matrine derivatives against Lipopolysaccharide (LPS)-induced inflammation model of RAW264.7 cells was detected using an NO assay kit. The Enzyme-Linked Immunosorbent Assay (ELISA) d was used to detect the secretion of Interleukin-6 (IL-6) and Tumor Necrosis Factor Alpha (TNF-α) by Matrine derivatives in LPS-induced inflammation model of RAW264.7 cells. Results The novel Matrine derivatives all exhibited lower cytotoxicity compared with M19. The NO inhibition rates of the novel Matrine derivatives were all higher than that of Matrine, and some were higher than that of M19 , with compound A12 having the highest NO inhibition rate. Compounds A11 and A12 showed higher IL-6 inhibition than the control M19 . Additionally, compound A12 had higher TNF-α inhibition than the control M19 . Conclusion Compound A12 inhibited the strongest inhibition of NO, IL-6 and TNF-α release and had the best anti-inflammatory activity, which provided an important lead compound for this subsequent in-depth study. -
表 1 苦参碱衍生物HR-MS和1H-NMR、13C-NMR数据
化合物 收率(%) HR-MS(ESI)
[M+H]+
计算值/理论值核磁数据(δ ppm) VI 76.3 294.1998 /294.1984 1H NMR(600 MHz, DMSO-d6)δ 5.41(dd, J=13.0, 4.3 Hz, 1H), 4.23–4.18(m, 1H), 3.62(s, 1H), 3.44–3.37(m, 3H), 3.14(dd, J=19.3, 4.4 Hz, 1H), 3.11–3.07(m, 1H), 3.06–2.99(m, 2H), 2.79–2.73(m, 2H), 2.64(dd, J=18.1, 10.0 Hz, 1H), 2.13(t, J=15.3 Hz, 2H), 2.03(dd, J=42.2, 12.1 Hz, 3H), 1.77(dd, J=24.1, 9.9 Hz, 4H), 1.67(t, J=14.9 Hz, 4H), 1.55(dd, J=15.9, 11.5 Hz, 1H). 13C NMR(151 MHz, DMSO-d6)δ 196.37, 62.94, 54.75, 54.57, 54.49, 48.81, 45.70, 44.70, 42.05, 33.55, 26.35, 26.08, 24.88, 23.42, 18.63, 18.05. A1 72.7 416.2166 /416.2169 1H NMR(600 MHz, CDCl3)δ 8.28(t, J=5.8 Hz, 1H), 7.77(d, J=7.9 Hz, 1H), 7.70(dd, J=9.5, 2.0 Hz, 1H), 7.39(td, J=8.0, 5.7 Hz, 1H), 7.17(td, J=8.3, 2.5 Hz, 1H), 5.80(dd, J=13.7, 4.4 Hz, 1H), 4.79(dd, J=17.6, 9.3 Hz, 1H), 3.64(dt, J=13.6, 5.6 Hz, 1H), 3.57(d, J=11.7 Hz, 1H), 3.53(d, J=11.9 Hz, 1H), 3.46(t, J=13.6 Hz, 1H), 3.20(s, 1H), 3.13(dt, J=18.4, 2.8 Hz, 1H), 2.97(dd, J=18.4, 5.1 Hz, 1H), 2.84(ddd, J=13.8, 11.1, 6.1 Hz, 1H), 2.75(t, J=12.8 Hz, 2H), 2.57(dd, J=10.4, 5.1 Hz, 1H), 2.34–2.23(m, 2H), 2.18(t, J=14.7 Hz, 2H), 2.07(d, J=14.7 Hz, 1H), 1.94(d, J=14.1 Hz, 1H), 1.89–1.85(m, 1H), 1.81(td, J=9.4, 4.5 Hz, 2H), 1.72(d, J=15.2 Hz, 1H), 1.61(dd, J=22.6, 12.2 Hz, 2H). 13C NMR(151 MHz, CDCl3)δ 199.37, 166.76, 162.81(d, J=246.7 Hz), 136.19(d, J=6.8 Hz), 130.10(d, J=7.8 Hz), 122.91, 118.46(d, J=21.3 Hz), 114.69(d, J=23.0 Hz), 64.82, 56.57, 56.42, 52.97, 48.34, 45.38, 42.13, 41.51, 33.84, 28.31, 27.94, 25.61, 24.36, 18.98, 18.49. A2 66.4 416.2166 /416.2166 1H NMR(600 MHz, CDCl3)δ 8.23(t, J=5.7 Hz, 1H), 7.98(dd, J=8.8, 5.3 Hz, 2H), 7.09(t, J=8.6 Hz, 2H), 5.79(dd, J=13.7, 4.4 Hz, 1H), 4.76(dd, J=17.4, 9.3 Hz, 1H), 3.61(dt, J=13.5, 5.8 Hz, 1H), 3.57(d, J=11.9 Hz, 1H), 3.53(d, J=11.5 Hz, 1H), 3.45(t, J=13.6 Hz, 1H), 3.21(s, 1H), 3.13(d, J=2.5 Hz, 1H), 2.98(dd, J=18.4, 5.0 Hz, 1H), 2.90–2.84(m, 1H), 2.76(t, J=12.7 Hz, 2H), 2.55(dd, J=12.0, 7.1 Hz, 1H), 2.29–2.22(m, 2H), 2.17(dd, J=17.9, 14.2 Hz, 2H), 2.06(d, J=14.4 Hz, 1H), 1.96–1.91(m, 1H), 1.90–1.86(m, 1H), 1.84–1.79(m, 2H), 1.72(d, J=15.0 Hz, 1H), 1.65–1.59(m, 2H). 13C NMR(151 MHz, CDCl3)δ 199.31, 167.48, 164.93(d, J=251.9 Hz), 129.82(d, J=8.7 Hz), 115.50(d, J=21.8 Hz), 64.84, 56.57, 56.42, 53.02, 48.36, 45.37, 42.09, 41.59, 33.84, 28.30, 28.04, 25.58, 24.31, 18.98, 18.49. A3 78.3 432.1871 /432.1900 1H NMR(600 MHz, CDCl3)δ 7.57(dd, J=7.5, 1.7 Hz, 1H), 7.38(d, J=6.6 Hz, 1H), 7.32(dt, J=16.2, 7.4 Hz, 3H), 5.71(d, J=9.2 Hz, 1H), 4.66(d, J=11.4 Hz, 1H), 3.65–3.49(m, 4H), 3.20(s, 1H), 3.11(d, J=18.4 Hz, 1H), 3.07–3.01(m, 1H), 2.96(d, J=18.6 Hz, 1H), 2.69(t, J=12.8 Hz, 2H), 2.50(s, 1H), 2.24(d, J=11.6 Hz, 1H), 2.13(dd, J=34.0, 17.2 Hz, 4H), 1.91(d, J=13.9 Hz, 2H), 1.79(dd, J=21.9, 11.8 Hz, 3H), 1.71(d, J=15.3 Hz, 1H), 1.61(t, J=14.4 Hz, 1H). 13C NMR(151 MHz, CDCl3)δ 198.84, 167.51, 135.31, 131.24, 130.58, 130.15, 129.75, 127.14, 64.90, 56.37, 56.20, 53.81, 48.80, 45.59, 42.52, 41.40, 34.03, 28.15, 27.97, 25.72, 24.55, 19.00, 18.52. A4 49.9 432.1871 /432.1892 1H NMR(600 MHz, CDCl3)δ 8.25(s, 1H), 7.96(s, 1H), 7.85(d, J=7.7 Hz, 1H), 7.45(d, J=8.0 Hz, 1H), 7.36(t, J=7.9 Hz, 1H), 5.79(dd, J=13.8, 4.3 Hz, 1H), 4.79(dd, J=17.1, 9.0 Hz, 1H), 3.65–3.60(m, 1H), 3.56(dd, J=20.0, 11.8 Hz, 2H), 3.47(t, J=13.5 Hz, 1H), 3.21(s, 1H), 3.12(d, J=18.3 Hz, 1H), 2.99(dd, J=18.4, 4.9 Hz, 1H), 2.91–2.85(m, 1H), 2.75(t, J=12.5 Hz, 2H), 2.56(d, J=4.4 Hz, 1H), 2.27(t, J=11.4 Hz, 2H), 2.17(dd, J=13.3, 4.4 Hz, 2H), 2.06(d, J =14.4 Hz, 1H), 1.93(d, J=14.3 Hz, 1H), 1.88(d, J=11.0 Hz, 1H), 1.80(td, J=14.6, 4.5 Hz, 2H), 1.72(d, J=15.2 Hz, 1H), 1.64–1.59(m, 2H). 13C NMR(151 MHz, CDCl3)δ 199.31, 166.92, 135.60, 134.71, 131.60, 129.83, 127.87, 125.38, 64.86, 56.56, 56.43, 53.05, 48.39, 45.39, 42.04, 41.66, 33.85, 28.29, 27.93, 25.61, 24.36, 18.98, 18.50. A5 54.7 432.1871 /432.1898 1H NMR(600 MHz, CDCl3)δ 8.22(s, 1H), 7.90(d, J=8.5 Hz, 2H), 7.40(d, J=8.5 Hz, 2H), 5.80(dd, J=13.9, 4.4 Hz, 1H), 4.76(dd, J=17.7, 8.7 Hz, 1H), 3.61(dd, J=12.7, 7.2 Hz, 2H), 3.56(d, J=11.3 Hz, 1H), 3.46(t, J=13.6 Hz, 1H), 3.22(s, 1H), 3.12(d, J=18.6 Hz, 1H), 2.99(dd, J=18.4, 5.1 Hz, 1H), 2.93(ddd, J=13.8, 10.8, 6.3 Hz, 1H), 2.76(t, J=12.7 Hz, 2H), 2.56(d, J=4.7 Hz, 1H), 2.30–2.22(m, 2H), 2.19–2.13(m, 2H), 2.06(s, 1H), 1.95(d, J=13.6 Hz, 1H), 1.90(d, J=11.5 Hz, 1H), 1.81(dd, J=10.4, 5.5 Hz, 2H), 1.73(d, J=15.0 Hz, 1H), 1.67–1.61(m, 2H). 13C NMR(151 MHz, CDCl3)δ 199.37, 167.77, 138.15, 131.81, 128.91, 128.86, 65.01, 56.64, 56.50, 53.08, 48.39, 45.31, 42.04, 41.81, 33.85, 28.31, 27.95, 25.61, 24.35, 19.00, 18.52. A6 53.4 476.1366 /476.1388 1H NMR(600 MHz, CDCl3)δ 7.57(dd, J=8.0, 0.9 Hz, 1H), 7.47(dd, J=7.6, 1.6 Hz, 1H), 7.34(td, J=7.5, 1.0 Hz, 1H), 7.28–7.25(m, 2H), 5.71(dd, J=13.5, 4.5 Hz, 1H), 4.65–4.58(m, 1H), 3.59(dd, J=20.0, 8.6 Hz, 2H), 3.56–3.46(m, 2H), 3.22(s, 1H), 3.12(dd, J=18.4, 5.1 Hz, 1H), 3.08–3.04(m, 1H), 2.95(dd, J=18.3, 6.2 Hz, 1H), 2.71(dd, J=16.9, 8.8 Hz, 2H), 2.47(s, 1H), 2.25(d, J=8.4 Hz, 1H), 2.21–2.16(m, 1H), 2.11(q, J=12.2 Hz, 3H), 1.96–1.88(m, 2H), 1.83(ddd, J=13.7, 6.6, 3.0 Hz, 1H), 1.80–1.75(m, 2H), 1.72(d, J=15.3 Hz, 1H), 1.63(ddd, J=19.0, 10.2, 4.4 Hz, 1H). 13C NMR(151 MHz, CDCl3)δ 198.81, 168.76, 137.61, 133.32, 131.36, 129.30, 127.66, 119.22, 64.95, 56.40, 56.22, 53.85, 48.78, 45.61, 42.58, 41.31, 34.00, 28.18, 28.05, 25.66, 24.52, 18.99, 18.54. A7 44.6 476.1366 /476.1384 1H NMR(600 MHz, CDCl3)δ 8.20(s, 1H), 8.09(s, 1H), 7.87(d, J=7.6 Hz, 1H), 7.62(d, J=7.8 Hz, 1H), 7.30(t, J=7.9 Hz, 1H), 5.78(dd, J=13.4, 3.7 Hz, 1H), 4.77(s, 1H), 3.58(dd, J=19.5, 10.7 Hz, 3H), 3.48(t, J=13.5 Hz, 1H), 3.23(s, 1H), 3.09(d, J=18.0 Hz, 1H), 3.03–2.98(m, 1H), 2.96–2.90(m, 1H), 2.76(s, 2H), 2.54(s, 1H), 2.29–2.23(m, 2H), 2.15(d, J=13.0 Hz, 2H), 2.06(d, J=14.5 Hz, 1H), 1.91(dd, J=22.6, 12.7 Hz, 2H), 1.82(d, J=14.2 Hz, 2H), 1.73(d, J=14.7 Hz, 1H), 1.67–1.61(m, 2H). 13C NMR(151 MHz, CDCl3)δ 167.14, 135.61, 134.69, 130.78, 130.15, 125.86, 122.79, 64.97, 56.59, 56.47, 53.14, 48.48, 45.44, 41.94, 41.87, 33.86, 28.27, 27.91, 25.62, 24.38, 18.99, 18.51. A8 44.6 412.2417 /412.2434 1H NMR(600 MHz, CDCl3)δ 8.01(s, 1H), 7.73(s, 1H), 7.70(d, J=4.0 Hz, 1H), 7.31(d, J=4.6 Hz, 2H), 5.77(dd, J=13.8, 4.2 Hz, 1H), 4.74(s, 1H), 3.57(dd, J=17.0, 11.6 Hz, 3H), 3.49(t, J=13.5 Hz, 1H), 3.22(s, 1H), 3.06–2.94(m, 3H), 2.75(t, J=12.3 Hz, 2H), 2.53(s, 1H), 2.38(s, 3H), 2.28–2.19(m, 2H), 2.13(dd, J=12.9, 6.9 Hz, 2H), 2.05(d, J=14.7 Hz, 1H), 1.91(t, J=15.0 Hz, 2H), 1.84–1.77(m, 2H), 1.65(ddd, J=24.5, 22.5, 14.1 Hz, 3H). 13C NMR(151 MHz, CDCl3)δ 199.13, 169.11, 138.51, 133.39, 132.63, 128.51, 128.02, 124.39, 64.92, 56.53, 56.38, 53.35, 48.54, 45.48, 42.07, 41.79, 33.91, 28.17, 27.99, 25.61, 24.34, 21.26, 18.99, 18.50. A9 72.9 443.2111 /443.2139 1H NMR(600 MHz, CDCl3)δ 8.79(t, J=1.7 Hz, 1H), 8.62(t, J=5.8 Hz, 1H), 8.33–8.29(m, 2H), 7.60(t, J=8.0 Hz, 1H), 5.80(dd, J=13.7, 4.5 Hz, 1H), 4.76(dd, J=17.3, 9.7 Hz, 1H), 3.68–3.63(m, 1H), 3.60–3.53(m, 2H), 3.44(t, J=13.6 Hz, 1H), 3.24(s, 1H), 3.14(d, J=18.4 Hz, 1H), 2.98(dd, J=18.4, 5.1 Hz, 1H), 2.92(ddd, J=13.8, 10.9, 6.1 Hz, 1H), 2.81–2.75(m, 2H), 2.56(d, J=5.1 Hz, 1H), 2.33–2.23(m, 2H), 2.22–2.12(m, 2H), 2.08(d, J=14.1 Hz, 1H), 1.92(t, J=15.5 Hz, 2H), 1.85–1.80(m, 2H), 1.74(d, J=14.8 Hz, 1H), 1.67–1.61(m, 2H). 13C NMR(151 MHz, CDCl3)δ 199.38, 166.00, 148.32, 135.64, 133.36, 129.71, 126.01, 122.74, 64.84, 56.60, 56.47, 52.95, 48.32, 45.27, 42.08, 41.58, 33.80, 28.45, 27.99, 25.56, 24.31, 18.97, 18.48. A10 40.9 443.2111 /443.2130 1H NMR(600 MHz, CDCl3)δ 8.59(s, 1H), 8.28–8.25(m, 2H), 8.20–8.18(m, 2H), 5.83(dd, J=13.8, 4.4 Hz, 1H), 4.84(dd, J=16.8, 10.1 Hz, 1H), 3.70(dt, J=13.7, 5.7 Hz, 1H), 3.56(dd, J=26.9, 11.8 Hz, 2H), 3.46(t, J=13.5 Hz, 1H), 3.24–3.18(m, 2H), 2.97(dd, J=18.4, 5.0 Hz, 1H), 2.88(ddd, J=13.9, 11.5, 6.0 Hz, 1H), 2.78(s, 2H), 2.61(s, 1H), 2.31–2.26(m, 2H), 2.20(dd, J=22.6, 10.4 Hz, 2H), 2.09(d, J=14.2 Hz, 1H), 1.97(d, J=14.0 Hz, 1H), 1.85(ddd, J=16.1, 11.5, 5.0 Hz, 3H), 1.74(d, J=14.8 Hz, 1H), 1.67–1.58(m, 2H). 13C NMR(151 MHz, CDCl3)δ 199.43, 166.28, 149.73, 139.20, 128.77, 123.69, 64.94, 56.63, 56.54, 52.78, 48.26, 45.29, 42.27, 41.52, 33.77, 28.42, 27.92, 25.58, 24.32, 18.98, 18.52. A11 50.7 454.2887 /454.2937 1H NMR(600 MHz, CDCl3)δ 8.00(t, J=5.5 Hz, 1H), 7.84(d, J=8.2 Hz, 2H), 7.23(d, J=8.2 Hz, 2H), 5.77(dd, J=13.7, 4.4 Hz, 1H), 4.75(dd, J=18.1, 7.5 Hz, 1H), 3.60–3.54(m, 3H), 3.50(t, J=13.5 Hz, 1H), 3.21(s, 1H), 3.08–2.93(m, 3H), 2.75(t, J=12.4 Hz, 2H), 2.65–2.62(m, 2H), 2.54(d, J=4.4 Hz, 1H), 2.22(dd, J=30.6, 14.0 Hz, 2H), 2.18–2.10(m, 2H), 2.05(d, J=14.9 Hz, 1H), 1.91(t, J=16.3 Hz, 2H), 1.84–1.77(m, 2H), 1.73–1.64(m, 2H), 1.60(dq, J=15.3, 7.7 Hz, 3H), 1.38–1.30(m, 2H), 0.92(t, J=7.4 Hz, 3H). 13C NMR(151 MHz, CDCl3)δ 199.22, 168.75, 147.33, 130.75, 128.66, 127.36, 64.91, 56.52, 56.38, 53.32, 48.50, 45.46, 41.99, 41.78, 35.56, 33.89, 33.31, 28.17, 27.98, 25.61, 24.35, 22.33, 18.99, 18.51, 13.91. A12 58.8 454.2887 /454.2908 1H NMR(600 MHz, CDCl3)δ 8.02(t, J=5.5 Hz, 1H), 7.88(d, J=8.4 Hz, 2H), 7.45(d, J=8.4 Hz, 2H), 5.79(dd, J=13.7, 4.4 Hz, 1H), 4.76(dd, J=18.4, 7.7 Hz, 1H), 3.61–3.55(m, 3H), 3.50(t, J=13.4 Hz, 1H), 3.21(s, 1H), 3.08(d, J=18.8 Hz, 1H), 3.01(dd, J=18.4, 4.9 Hz, 1H), 2.93(ddd, J=13.8, 10.5, 6.1 Hz, 1H), 2.74(t, J=12.6 Hz, 2H), 2.56(dd, J=9.6, 4.8 Hz, 1H), 2.28–2.13(m, 4H), 2.05(d, J=14.7 Hz, 1H), 1.91(dd, J=26.8, 12.8 Hz, 2H), 1.80(ddd, J=14.0, 9.4, 4.3 Hz, 2H), 1.71(d, J=15.1 Hz, 1H), 1.66–1.57(m, 2H), 1.32(s, 9H). 13C NMR(151 MHz, CDCl3)δ 199.32, 168.54, 155.37, 130.56, 127.20, 125.56, 64.93, 56.53, 56.40, 53.25, 48.47, 45.45, 41.89, 41.85, 34.98, 33.88, 31.18, 28.18, 27.95, 25.63, 24.38, 18.99, 18.53. A13 69.5 423.2213 /423.2241 1H NMR(600 MHz, CDCl3)δ 8.54(t, J=5.8 Hz, 1H), 8.32(s, 1H), 8.24(d, J=8.0 Hz, 1H), 7.76(d, J=7.7 Hz, 1H), 7.55(t, J=7.8 Hz, 1H), 5.81(dd, J=13.7, 4.5 Hz, 1H), 4.79(dd, J=17.0, 9.8 Hz, 1H), 3.69–3.64(m, 1H), 3.55(dd, J=22.9, 11.4 Hz, 2H), 3.44(t, J=13.6 Hz, 1H), 3.23(s, 1H), 3.15(d, J=18.4 Hz, 1H), 2.98(dd, J=18.4, 5.0 Hz, 1H), 2.90–2.83(m, 1H), 2.78(s, 2H), 2.56(d, J=5.2 Hz, 1H), 2.29(t, J=13.5 Hz, 2H), 2.23–2.14(m, 2H), 2.08(d, J=14.5 Hz, 1H), 1.95(d, J=14.1 Hz, 1H), 1.90(d, J=11.0 Hz, 1H), 1.83(ddd, J=14.3, 9.7, 4.6 Hz, 2H), 1.75(d, J=15.1 Hz, 1H), 1.64(dt, J=19.4, 6.9 Hz, 2H). 13C NMR(151 MHz, CDCl3)δ 199.30, 166.10, 135.00, 134.72, 131.72, 131.45, 129.46, 118.20, 112.80, 64.85, 56.61, 56.48, 52.91, 48.31, 45.30, 42.17, 41.53, 33.80, 28.34, 28.01, 25.57, 24.29, 18.97, 18.50. A14 74.0 423.2213 /423.2242 1H NMR(600 MHz, CDCl3)δ 8.57(t, J=5.7 Hz, 1H), 8.12(d, J=8.4 Hz, 2H), 7.71(d, J=8.5 Hz, 2H), 5.82(dd, J=13.8, 4.4 Hz, 1H), 4.79(dd, J=17.0, 10.3 Hz, 1H), 3.71–3.66(m, 1H), 3.56(d, J=11.7 Hz, 1H), 3.51(d, J=11.9 Hz, 1H), 3.41(t, J=13.6 Hz, 1H), 3.24–3.15(m, 2H), 2.95(dd, J=18.4, 5.1 Hz, 1H), 2.82(ddd, J=25.2, 14.4, 8.7 Hz, 3H), 2.58(s, 1H), 2.29(dd, J=28.5, 14.4 Hz, 2H), 2.24–2.13(m, 2H), 2.07(d, J=14.5 Hz, 1H), 1.95(d, J=14.2 Hz, 1H), 1.90–1.79(m, 3H), 1.73(d, J=14.8 Hz, 1H), 1.66–1.56(m, 2H). 13C NMR(151 MHz, CDCl3)δ 199.34, 166.36, 137.67, 132.30, 128.20, 118.27, 114.95, 64.81, 56.61, 56.47, 52.74, 48.21, 45.27, 42.30, 41.38, 33.76, 28.37, 27.98, 25.55, 24.27, 18.96, 18.49. 表 2 苦参碱及其衍生物对 RAW 264.7的细胞毒性(n=3)
化合物 细胞存活率(%) 3.12 μmol/L 6.25 μmol/L 12.5 μmol/L 25 μmol/L 50 μmol/L 苦参碱 99.42±0.19 97.24±0.42 96.29±0.59 94.24±0.28 89.13±0.06 M19 94.09±0.57 92.76±0.41 90.74±0.20 80.66±0.38 52.87±0.41 VI 98.10±1.04 97.25±0.78 96.73±0.61 96.29±0.92 95.19±0.59 A1 99.50±0.23 98.71±0.46 96.21±0.93 94.19±0.45 91.85±0.76 A2 99.60±0.21 98.81±0.54 96.71±0.51 93.78±1.18 90.77±0.81 A3 98.89±0.77 96.84±0.86 93.96±0.59 93.20±0.46 90.52±1.07 A4 99.84±0.16 98.69±0.38 97.59±0.92 95.59±1.19 94.35±0.81 A5 99.64±0.28 98.73±0.25 97.73±0.93 94.52±0.53 90.92±0.71 A6 98.75±0.86 95.81±0.61 92.74±0.28 88.37±1.23 83.62±0.52 A7 96.01±1.04 94.42±0.47 92.40±0.37 83.15±0.47 68.15±0.82 A8 95.93±0.75 94.49±0.96 92.97±1.18 83.94±1.21 76.86±0.72 A9 99.40±0.16 94.93±0.30 92.33±0.23 88.89±0.39 75.29±0.57 A10 99.20±0.27 96.31±0.57 94.89±0.29 86.78±1.22 80.82±1.23 A11 97.87±0.22 95.87±0.70 92.81±0.29 86.60±0.61 55.31±0.84 A12 97.88±0.44 94.68±0.59 91.92±0.46 83.68±0.39 76.95±0.74 A13 97.57±0.54 95.36±1.21 94.95±0.53 83.86±0.66 81.46±0.33 A14 95.59±0.70 94.76±0.64 92.90±0.57 82.33±0.73 78.85±0.66 表 3 苦参碱衍生物在12.5 μmol/L下对LPS诱导的RAW264.7细胞的NO释放的影响(n=3)
化合物 NO抑制率(%) 化合物 NO抑制率(%) 苦参碱 40.95±0.73 A7 57.33±0.53### M19 64.39±0.66### A8 55.14±0.34### VI 55.93±0.75### A9 58.92±0.10### A1 56.62±0.73### A10 69.40±0.28*** A2 57.13±0.25### A11 67.50±0.44*** A3 60.01±0.53### A12 72.70±0.58*** A4 61.27±0.24### A13 61.31±0.36### A5 63.61±0.38### A14 63.62±0.37### A6 67.22±0.99** **p<0.01, ***p<0.001 与M19相比; ###p<0.001 与苦参碱相比 表 4 苦参碱衍生物在12.5 μmol/L下对LPS诱导的RAW264.7细胞的IL-6和TNF-α释放的影响(n=3)
化合物 IL-6 抑制率(%) TNF-α 抑制率(%) M19 59.77±0.39 49.57±0.92 A6 63.18±0.48*** 56.7±1.02*** A10 69.84±0.32*** 52.2±1.36* A11 86.21±0.51*** 55.46±0.61*** A12 86.64±0.33*** 71.24±1.25*** *p<0.05, **p<0.01, ***p<0.001与 M19相比 -
[1] RALLIS M, KYRIAZI M, PAPAIOANNOU G T, et al. P 152 skin inflammation and oxidative stress[J]. Free Radic Biol Med, 2017, 108:S70. [2] HWANG S J, SONG Y S, LEE H J. Phaseolin attenuates lipopolysaccharide-induced inflammation in RAW 264.7 cells and zebrafish[J]. Biomedicines, 2021, 9(4):420. doi: 10.3390/biomedicines9040420 [3] SCRIVO R, VASILE M, BARTOSIEWICZ I, et al. Inflammation as “common soil” of the multifactorial diseases[J]. Autoimmun Rev, 2011, 10(7):369-374. doi: 10.1016/j.autrev.2010.12.006 [4] ZHANG W F, XIAO D, MAO Q W, et al. Role of neuroinflammation in neurodegeneration development[J]. Signal Transduct Target Ther, 2023, 8(1):267. doi: 10.1038/s41392-023-01486-5 [5] FALCONER J, MURPHY A N, YOUNG S P, et al. Review: synovial cell metabolism and chronic inflammation in rheumatoid arthritis[J]. Arthritis Rheumatol, 2018, 70(7):984-999. doi: 10.1002/art.40504 [6] SOTÁK M, CLARK M, SUUR B E, et al. Inflammation and resolution in obesity[J]. Nat Rev Endocrinol, 2025, 21:45-61. doi: 10.1038/s41574-024-01047-y [7] GRAINGE C, PARK J A. Inflammatory insights into airway remodelling in asthma[J]. Respirology, 2018, 23(12):1084-1085. doi: 10.1111/resp.13390 [8] UYAR B, PALMER D, KOWALD A, et al. Single-cell analyses of aging, inflammation and senescence[J]. Ageing Res Rev, 2020, 64:101156. doi: 10.1016/j.arr.2020.101156 [9] 张宇实, 丛伟红, 张晶晶, 等. 中草药及其活性成分对人冠状病毒干预作用的研究进展[J]. 中国中药杂志, 2020, 45(6):1263-1271. [10] 席宇, 申光焕, 崔琳琳, 等. 苦参碱类衍生物的合成及其活性研究进展[J]. 合成化学, 2024, 32(4):381-392. [11] 杨守研, 刘瑛琦. 苦参的化学成分、药理作用及临床应用研究进展[J]. 中国药物滥用防治杂志, 2024, 30(1):80-83. [12] 张雨, 苏丹丹, 王亚男, 等. 旱生植物苦豆子研究综述[J]. 中国野生植物资源, 2021, 40(9):55-58. doi: 10.3969/j.issn.1006-9690.2021.09.010 [13] 赵宝中, 荣大奇, 王秀军, 等. 苦参碱和氧化苦参碱电子结构与药性的关系[J]. 分子科学学报, 2000, 16(2):88-93. doi: 10.3969/j.issn.1000-9035.2000.02.005 [14] 詹原尧. 苦参生物碱—苦参碱和氧化 苦参碱的提取、分离方法研究[J]. 天津药学, 1991, 3(3):7-10. [15] HU H G, WANG S Z, ZHANG C M, et al. Synthesis and in vitro inhibitory activity of matrine derivatives towards pro-inflammatory cytokines[J]. Bioorg Med Chem Lett, 2010, 20(24):7537-7539. doi: 10.1016/j.bmcl.2010.09.075 [16] 孙云龙. 水杨酸类苦参碱衍生物的合成及其抗炎和抗肿瘤活性研究[D]. 南宁: 广西大学, 2014. [17] 李本鹏. NO供体苦参碱衍生物的设计合成及其抗心肌缺血保护作用的初步研究[D]. 银川: 宁夏医科大学, 2015. [18] LUO D, LIN Q, TAN J L, et al. Water-soluble matrine-type alkaloids with potential anti-neuroinflammatory activities from the seeds of Sophora alopecuroides[J]. Bioorg Chem, 2021, 116:105337. doi: 10.1016/j.bioorg.2021.105337 [19] 冯轶. 新型苦参碱衍生物抑制肝星状细胞活化及抗肝纤维化机制的实验研究[D]. 南京: 南京医科大学, 2016. -




 下载:
下载: