-
苦参为豆科植物苦参(Sophora flavescens Ait.)的干燥根,春秋二季采挖,除去根头及支根后洗净、干燥,或趁鲜切片、干燥,是我国传统中药,在全国大部分地区均产;味苦,性寒,归心、肝、胃、大肠、膀胱经,具有清热解毒、燥湿、杀虫、利尿及抗炎镇痛的功效,能够用于治疗热痢、便血、黄疸尿闭、赤白带下、皮肤瘙痒、湿疹、湿疮等症状,最早记载于《神农本草经》[1]。苦参的多种复方制剂已在临床上广泛应用,其中主要有效成分包括苦参碱和氧化苦参碱[2]。现代药理学研究表明,苦参具有抗炎、抗肿瘤、抗心律失常以及抗病原微生物等多种药理作用[3]。本文将对苦参的化学成分、药理作用、复方制剂及苦参碱的结构修饰进展进行综述,以期为苦参的临床应用及新药研发提供理论依据。
-
现代研究表明,苦参因含丰富的化学成分而具有多种药理作用,其中苦参碱和氧化苦参碱作为中药苦参最主要的活性成分,在抗炎、抗肿瘤、抗心律失常、抗病原微生物等多方面作用显著。现将苦参碱和氧化苦参碱的药理作用进行概述。
-
苦参碱和氧化苦参碱对多种慢性或急性炎症均具有良好的抗炎作用[3,5]。有研究表明,苦参碱能够抑制脂多糖(LPS)诱导的巨噬细胞分泌白细胞介素-6(IL-6)、IL-1β和肿瘤坏死因子(TNF-α)等炎症因子[6];苦参碱抑制炎症因子产生与其抑制核转录因子κB(NF-κB)和丝裂原活化蛋白激酶(MAPK)信号通路有关。苦参碱抑制促炎因子的产生,可预防无乳链球菌感染引起的细胞损伤[7]。苦参碱还可以改善阿尔兹海默症小鼠的学习和记忆障碍以及神经炎症,这一作用主要是通过抑制对海马组织中小胶质细胞活化和烟酰胺腺嘌呤二核苷酸磷酸氧化酶(NOX)的表达来实现的[8]。过敏性接触性皮炎(ACD)是一种高度流行的炎症和免疫性皮肤病,常伴有持续的疼痛和瘙痒,氧化苦参碱可通过抑制环氧化酶、炎症介质前列腺素的合成以及花生四烯酸的代谢转化来产生镇痛、止痒和抗炎作用[9]。此外,苦参碱可通过抑制NF-κB信号通路,减少炎症和改善肺血管重塑,逆转肺动脉平滑肌细胞的增殖和凋亡的不平衡,从而治疗缺氧性肺动脉高压[10]。
-
苦参碱和氧化苦参碱作为中药苦参的主要有效成分,可通过多种途径发挥抗肿瘤作用。有研究发现氧化苦参碱通过增加肿瘤细胞G0/G1期、减少S期,从而使肿瘤细胞的增殖停留在G2/M期,达到抑制肿瘤细胞增殖的作用[11]。另外苦参碱还可以增强非编码小RNA 分子miR-22的表达,阻断丝裂原活化的细胞外信号调节激酶/细胞外调节蛋白激酶(MEK/ERK)信号通路,促进结肠癌细胞的凋亡并抑制结肠癌细胞的进一步生长[12]。细胞侵袭和迁移是肿瘤的主要生物学特征之一[13],也是临床上治疗肿瘤的难点,氧化苦参碱能够参与调节多种肿瘤细胞的侵袭性转移[14],其中最有可能通过激活NF-κB信号通路达到抗肿瘤作用[15]。端粒酶作为一种基本的核蛋白反转录酶,能够控制端粒的长度,端粒酶活性在正常细胞中较低,在癌细胞中上调,导致无限的细胞增殖和肿瘤生长[16],有结果显示苦参碱能够剂量依赖的降低端粒酶的活性,因此苦参的抗肿瘤作用还可能与端粒酶有关[17]。
有研究发现,肝癌细胞经苦参碱处理后数量逐渐减少,且形态逐渐变圆,开始出现不完整的细胞,甚至出现细胞碎片,同时与调控肿瘤细胞增殖、发育和死亡相关的信号通路ERK1/2-MAPK表达显著下调,这也表明了苦参碱可通过该信号通路抑制肝癌细胞的存活[18-19]。有实验研究氧化苦参碱对5-氟尿嘧啶耐药结肠癌细胞的体外致敏情况,结果表明,氧化苦参碱可以调节肿瘤细胞的上皮-间质转化并抑制NF-κB信号通路,进而作为一种潜在药物来改善5-氟尿嘧啶化学耐药性[20]。
-
心律失常是心血管疾病中非常重要的一种疾病,是由心脏起搏和传导功能障碍引起的心跳节律和频率异常,在中医学中主要表现为心悸,是心血管疾病的常见症状和多发病之一[21]。《神农本草经》中有记载:“苦参…主心腹气结,癥瘕积聚,黄疸,溺有余沥,逐水,除痈肿。”《名医别录》有关于苦参具有宁神定悸功效的记载:“苦参…养肝胆气,安五脏,定志,益精,利九窍”。
研究表明,氧化苦参碱预处理心室肌细胞后,不仅能够抑制乌头碱诱导的大鼠心律失常,还能延缓心律失常的发作、缩短持续时间,降低死亡率[22]。病理性心脏纤维化也是心血管疾病的常见特征[23]。苦参碱能够显著抑制心脏成纤维细胞的增殖、迁移及胶原蛋白的产生,可能是通过调节小鼠的核糖体蛋白S5/p38丝裂原活化蛋白激酶(RPS5/p38 MAPK)信号转导来实现这一作用,从而减轻心脏纤维化[24]。在氧化苦参碱缓解心律失常的过程中,还伴随着多种离子通道的变化,氧化苦参碱通过降低L型钙电流(ICa,L)、增强瞬时外向钾电流(Ito)和抑制内向整流钾电流(IK1)来缩短大鼠心室肌细胞动作电位的持续时间[25]。在体内氧化苦参碱经进一步的代谢可以转化为苦参碱,二者均具有通过调节钠电流和钙电流通道达到抗心律失常的作用[26]。
-
苦参具有广谱抗菌活性,其有效成分对多种细菌和真菌微生物,如大肠杆菌、烟曲霉菌具有明显的抑制作用[27-28]。有结果表明,氧化苦参碱能够通过抑制真菌烟曲霉的生长、生物膜的形成、真菌细胞的完整性和分生孢子的黏附能力来降低真菌的负荷能力以及产生的相应炎症反应[27]。另一结果发现细菌生物膜的形成能力与耐药性之间呈正相关,苦参碱能够通过调控群体的感应系统来抑制大肠杆菌菌株生物膜的形成进而发挥较好的抗菌效果[28]。
-
复方苦参注射液(CKI)是一种中成药制剂,主要是由苦参和土茯苓,以及醋酸、氢氧化钠、聚山梨酯80等辅料经过一系列现代制剂工艺加工制成,主要成分包括黄酮类、生物碱类、糖苷类以及酚酸类等,具有清热利湿、凉血解毒、散瘀止痛的功效,可用于抗炎[29]、抗肿瘤[30]和抗纤维化[31]。
抗肿瘤作用是CKI主要的临床应用。研究显示,CKI可以联合不同的化疗方案治疗晚期结肠癌,具有协同治疗效果、减轻副作用、缓解疼痛和治疗癌症腹水的作用,且其安全性良好[32]。苦参碱和氧化苦参碱是CKI中的主要活性成分,CKI与化疗联合治疗胃癌的过程中,氧化苦参碱可能通过作用于TGFβ Ⅱ型受体(TβRⅡ),进而调节TGFβ/Smad信号通路,控制细胞周期并抑制胃癌细胞的增殖[33];另一项研究显示,CKI通过调控TNF信号通路诱导的血管细胞黏附分子-1(VCAM1)来抑制胃癌的上皮-间充质样细胞转化,有效抑制胃癌细胞的生长和转移[34]。CKI能够降低放射性肺炎患者的辐射损伤程度和毒性,这一作用可能与CKI有效成分苦参碱和氧化苦参碱的抗氧化作用有关,另有研究显示,CKI通过阻断瞬时受体电位香草酸亚型1(TRPV1)抑制ERK减少肿瘤相关促炎细胞因子的产生,并减轻癌症相关疼痛[35-36]。
-
苦参凝胶来源于《金匮要略》中的苦参汤,是由苦参中的苦参总碱制成,制剂组成较为简单,与复方制剂相比较具有质量稳定可控、疗效明确、无药物间相互作用、不良反应较少的优势,且水凝胶是一种聚合物在水中溶胀形成的药物传递系统,具有较高的含水量,适合用于黏膜、皮肤等给药途径[37]。苦参凝胶可在阴道内长时间滞留,具有抗菌、保护阴道黏膜等优点,是治疗妇科慢性炎症的理想药物[38]。苦参凝胶与氟康唑胶囊联合使用能够提高药物的抗真菌活性,治疗霉菌性阴道炎[39];在临床上对慢性宫颈炎患者采用苦参凝胶进行术后治疗,能够显著提升患者的治疗效果,促进患者进一步康复[40]。
-
在临床上,苦参相关复方制剂还可以与其他制剂联合使用,以发挥更强的抗炎、抗病原微生物等作用。当归苦参丸与克林霉素磷酸酯凝胶联合应用能够治疗累及面部及毛囊皮脂腺的炎症性皮肤疾病,有效降低炎症反应[41];五味苦参胶囊联合水杨酸制剂治疗溃疡性结肠炎,抑制炎症因子表达,增强肠道屏障功能[42];复方苦参止痒软膏联合糠酸莫米松乳膏治疗慢性湿疹的效果显著,患者的症状与炎症表现均有明显的改善,同时增强人体的免疫功能,值得临床推广应用[43]。
-
苦参碱具有抗肿瘤、抗纤维化、抗炎、抗心律失常等多种药理活性[44],然而其临床应用还存在一些问题,例如苦参碱的活性相对较弱,在体外的有效浓度为50~200 μmol/L;脂溶性较低,生物利用度差,体内半衰期短,一项关于苦参碱在大鼠中药代动力学的研究表明,苦参碱的生物利用度仅为18.5%;苦参碱的毒性最常见的是肝毒性和神经毒性[45-46]。有研究表明,氧化苦参碱和苦参碱对小鼠均有一定的毒性,但氧化苦参碱的毒性明显弱于苦参碱,推测其毒性差异可能与苦参碱环状结构中的叔胺结构氧化为季胺有关,故有必要对苦参碱进一步进行结构改造和化学修饰,以获得更好的生物活性[47-48]。
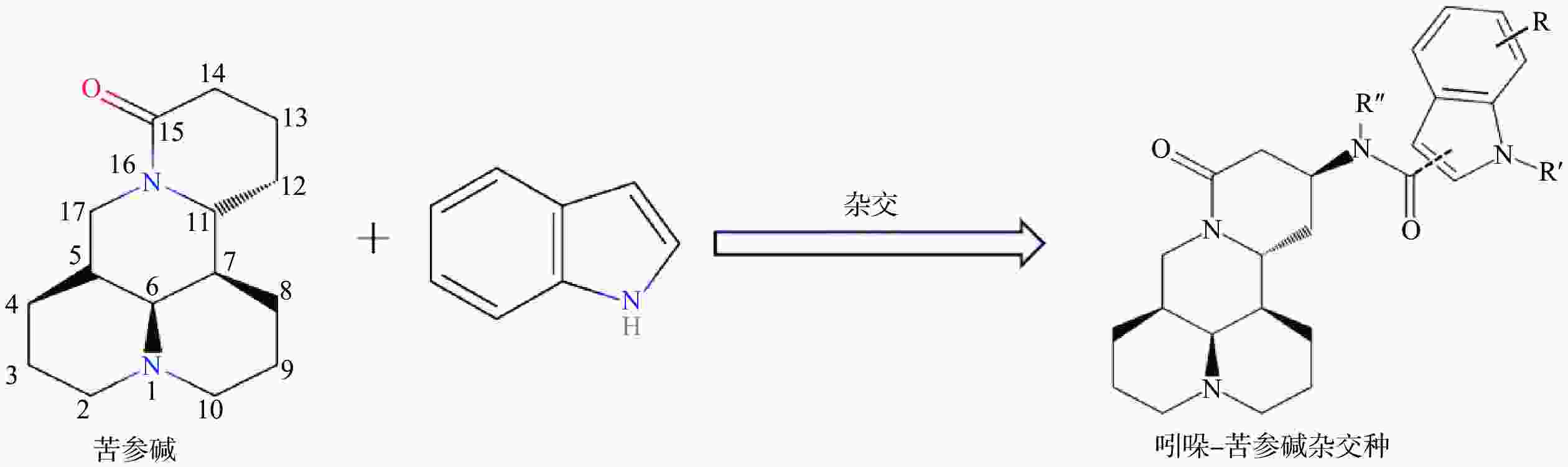
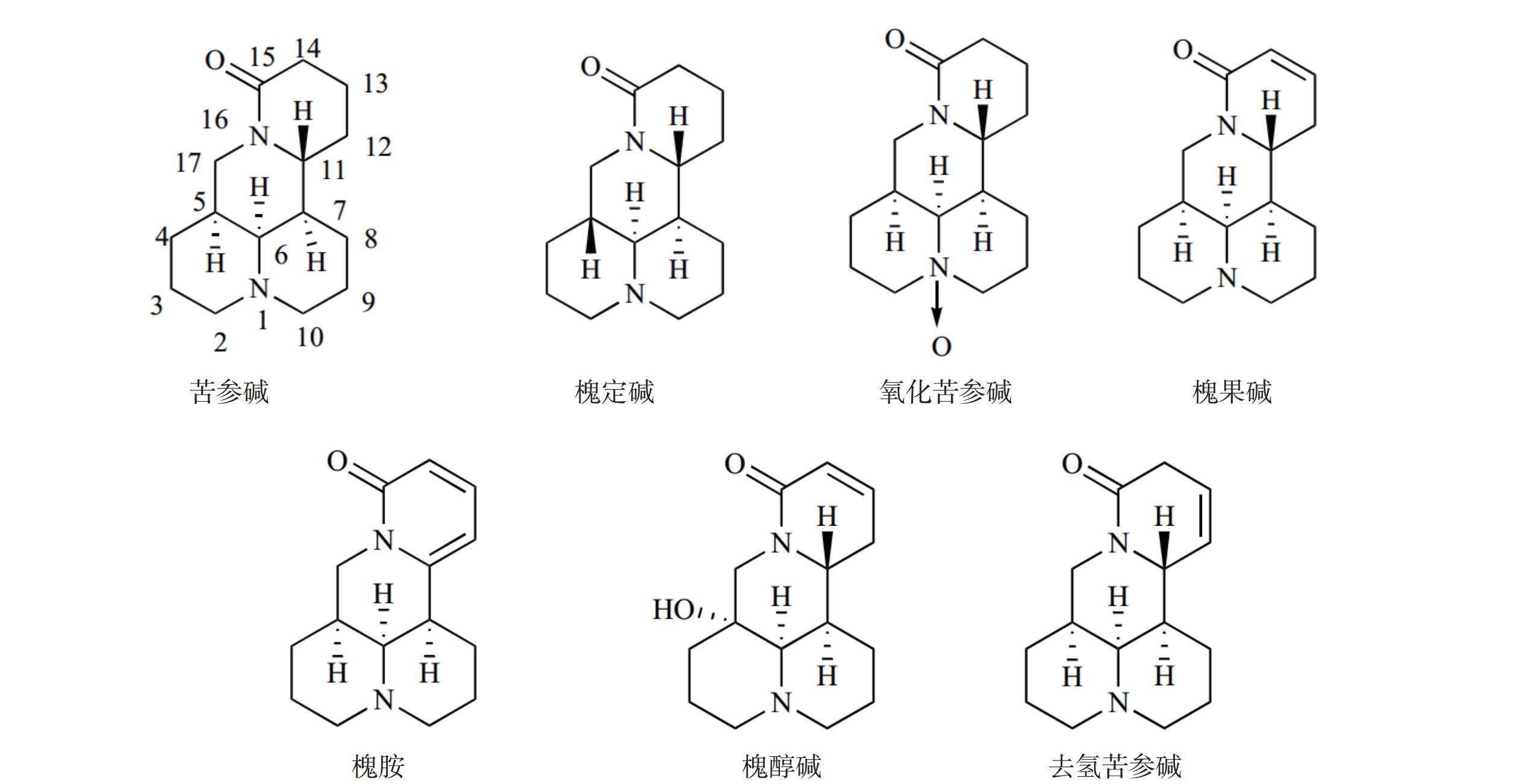
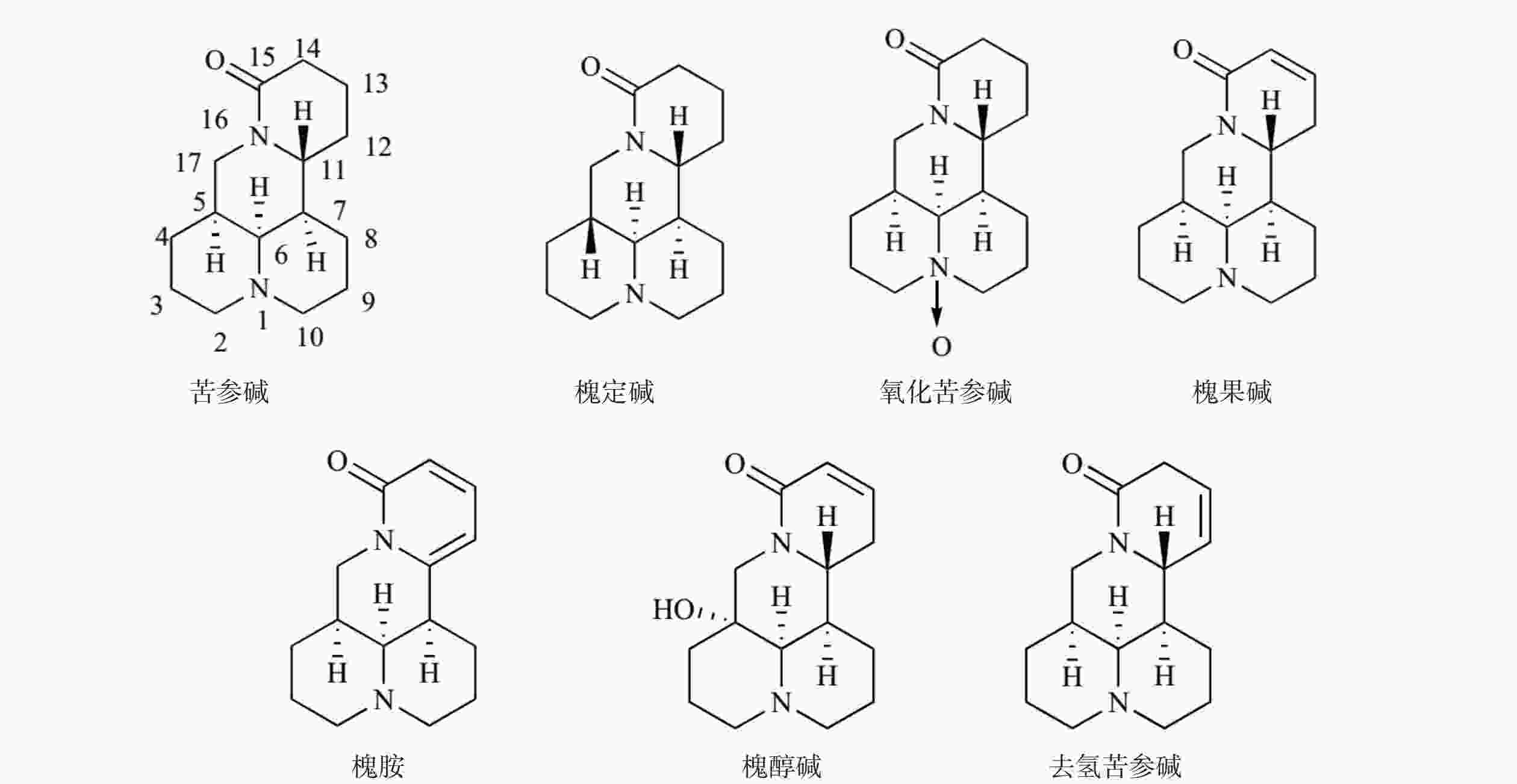
苦参碱(C15H24N2O)具有独特的双喹啉四环结构,包含有A/B喹啉环和C/D喹啉环两个不对称的喹啉片段(图1)。近年来,药物学专家在研究苦参碱衍生物时,常以苦参碱的C-13、C-14、C-15及D环开环等方面作为切入点来进行苦参碱的结构改造。
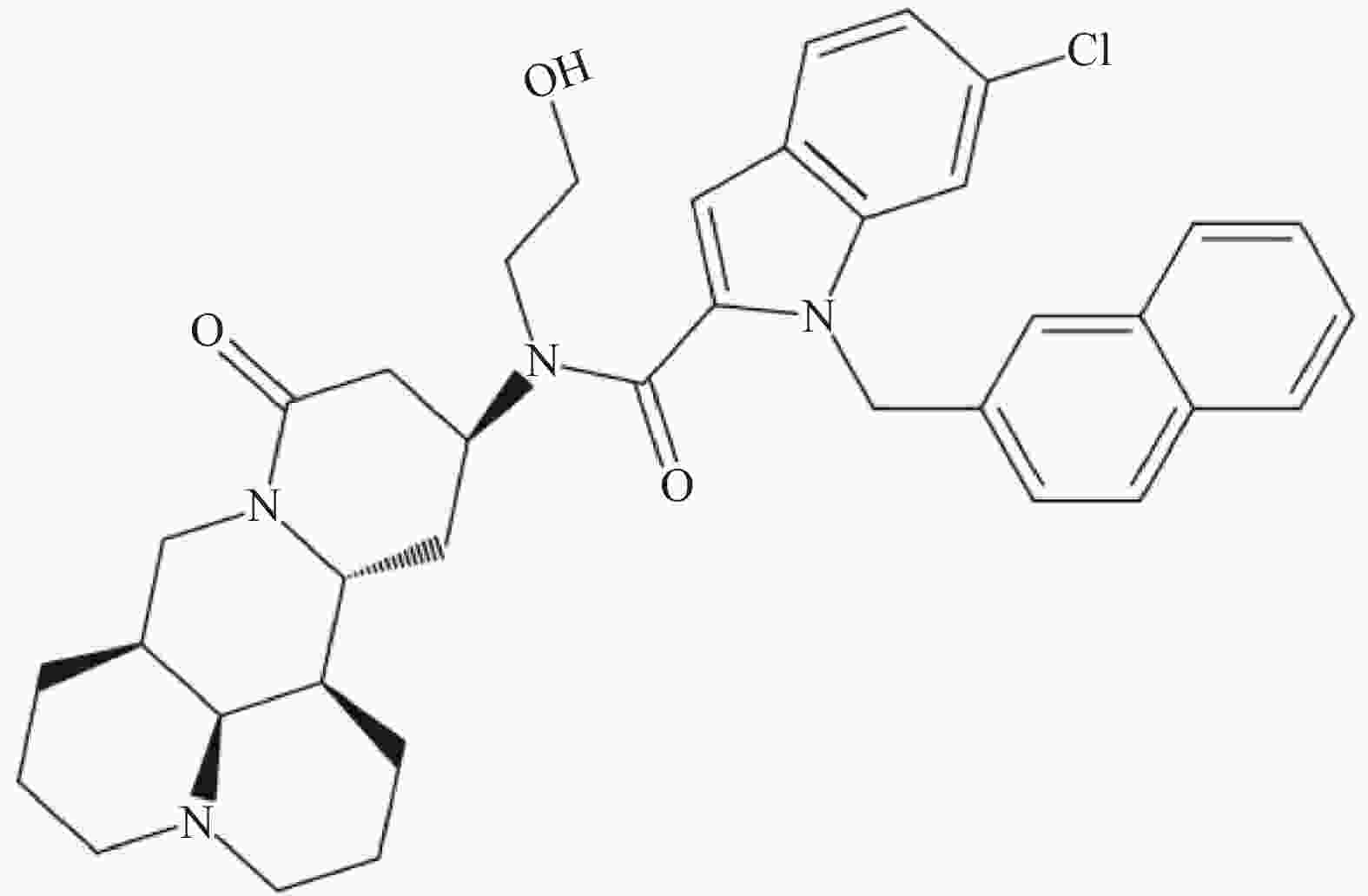
C-13位是提高苦参碱药理活性最常见的修饰位点之一,Li等[49]研究发现在苦参碱的C-13位引入吲哚支架,设计合成一系列吲哚-苦参碱杂交种(图2),其中杂交种(8g)(图3)的抗癌活性和对癌细胞的选择指数显著高于苦参碱,进一步研究发现(8g)靶向作用于线粒体,可破坏细胞内的能量供应,抑制癌细胞的增殖,因此可作为一种重要的潜在抗癌剂。
有研究显示,在苦参碱的C-14位引入苯甲酰基,进而合成一系列苦参碱衍生物,其中大部分苦参碱衍生物对多种癌细胞,如肺癌细胞A594、乳腺癌细胞MCF-7、胃癌细胞SGC-7901和肝癌细胞Bel-7402的IC50值比苦参碱低17~109倍,并且抑制肿瘤细胞的迁移,展现出更强的抗癌作用[50]。
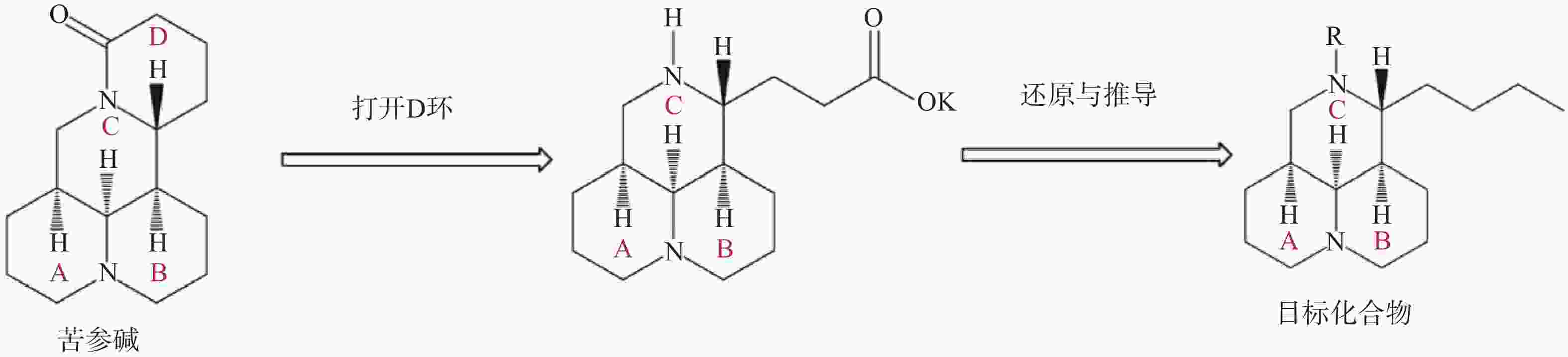
苦参碱D环打开后,可将C-11侧链还原为丁基,并进一步在氮原子上引入磺酰基、酰基或烷基等,得到多种苦参碱衍生物(图4)。与苦参碱相比,这些化合物在体内和体外均表现出良好的抗烟草花叶病毒(TMV)活性,这可能是与氮及侧链上的取代基有关[51]。
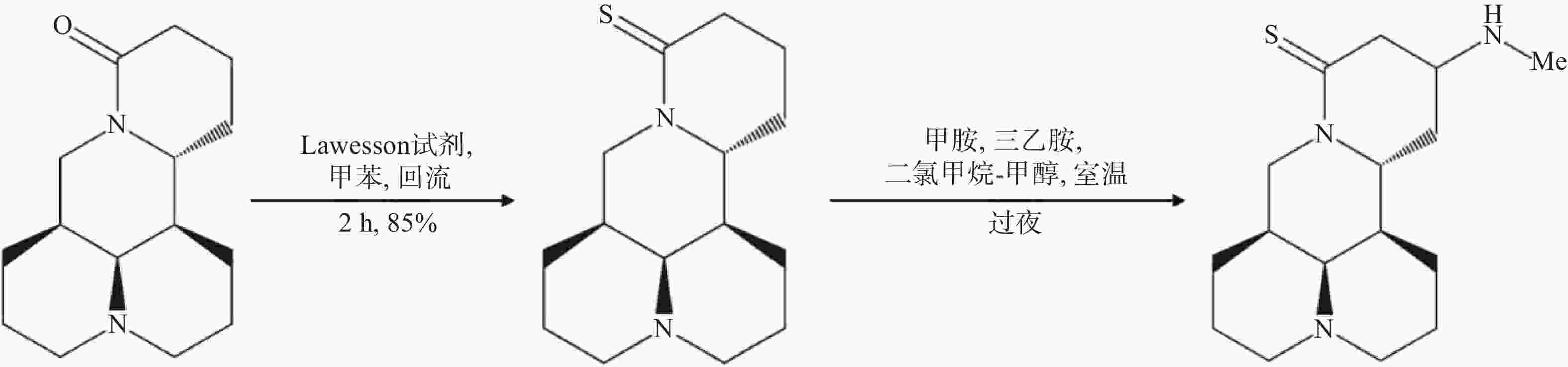
苦参碱结构改造方法还包括将苦参碱转化为硫代苦参碱,并在酮β位引入多种氨基,合成一系列苦参碱衍生物,其中,Hu等[52]合成的MASM(C16N3H27S),又称M19,即(6aS, 10S, 11aR, 11bR, 11cS)-10甲氨基-十二氢-3a, 7-二氮杂苯并 [de] 蒽-8-硫酮(图5),具有较好的药理作用,与苦参碱相比具有更好的抗炎和抗纤维化的作用以及更低的毒性和不良反应[53]。MASM的体外有效作用浓度为1~20 μmol/L;MASM的抗炎活性较苦参碱和槐果碱相比也有显著提高,1~10 mg/kg的MASM抗炎和抗纤维化作用与30~100 mg/kg的苦参碱相当[54]。有研究显示,MASM经灌胃给药后约1.3 h即可达到血浆药物峰值浓度,与苦参碱达峰时间2.1 h相比,口服MASM可以快速吸收,更快地发挥药理作用[55];毒性实验结果显示,MASM对小鼠的体重、肝肾等器官重量和生化指标的不良影响均很小或无影响,表明了MASM的无毒性或低毒性[55]。另有结果表明,脓毒症发作后仅使用1 mg/kg的MASM治疗即可减少全身和组织炎症反应,减轻多器官损伤,提高总生存率,这可能是与RPS5介导的抑制NF-κB和MAPK的激活以及降低促炎介质的表达有关[47];MASM还能够抑制星形胶质细胞激活,降低其炎症反应,改善血脑屏障功能,维持星形胶质细胞的功能,具有显著缓解免疫性脑脊髓炎发展的功能[54];此外MASM能够显著抑制肝癌细胞的增殖,诱导细胞凋亡和生长停滞,有望为治疗肝细胞癌提供新的治疗方法[56]。
-
苦参作为临床上常用的中药,应用广泛,且在《神农本草经》中具有重要的地位,在现代临床研究中,苦参能够具有很高的药用价值和科研价值主要是其包含苦参碱和氧化苦参碱等有效成分。然而,由于苦参中有效成分的活性相对较低,生物利用度差且存在毒性作用,严重影响了苦参的药效,因此可以将苦参碱进行结构修饰来增强药物的活性、降低用量和药物的毒性作用。目前,对于苦参化学成分及药理作用的研究有了一定的进展,但一些机制研究还有待进一步完善,同时苦参碱衍生物的深入研究对中药苦参的进一步开发和利用也具有重要意义。
Research progress on Sophora Flavescens of traditional Chinese medicine
-
摘要: 中药苦参为豆科植物苦参的干燥根,始载于《神农本草经》。苦参中含有多种活性成分,主要包括苦参碱和氧化苦参碱,具有抗炎、抗肿瘤、抗心律失常与抗病原微生物等多种药理作用,临床上苦参复方制剂主要包括复方苦参注射液、苦参凝胶等,可用于治疗多种癌症,改善皮肤、黏膜瘙痒疼痛等多种症状。由于苦参碱生物利用度较差,因此需要对苦参碱进行结构改造,其中,苦参碱衍生物MASM仅需较低浓度即可对脓毒症和肝纤维化等疾病具有较好的治疗效果。该文主要对苦参的化学成分、药理作用、复方制剂及苦参碱的结构修饰进展进行综述,为中药苦参的临床应用和新药研发提供理论依据。Abstract: Sophora Flavescens is the dried root of the leguminous plant Sophora Flavescens Ait. It was first published in Shen Nong's Herbal Classic. Sophora Flavescens contains a variety of active ingredients, mainly including matrine and oxymatrine, with anti-inflammatory, anti-tumor, anti-arrhythmia, disease-resistant pathogenic microorganisms and other pharmacological effects. Clinically, the compound preparations of Sophora Flavescens include Compound KuShen injection and KuShen gel and so on, which can be used to treat many types of cancers and improve skin, mucous pruritus, pain and other symptoms. Due to the poor bioavailability, the structure of matrine needs to be reformed. MASM, matrine derivative, only needs a low concentration to have a good therapeutic effect on sepsis and liver fibrosis. In this article, the chemical composition, pharmacological effects, compound preparations and structural modification of matrine were mainly discussed, aiming to provide a theoretical basis for the clinical application of Sophora Flavescens and the development of new drugs.
-
Key words:
- Sophora flavescens Ait /
- matrine /
- oxymatrine /
- pharmacological effect /
- MASM
-
[1] 国家药典委员会. 中华人民共和国药典(一部) 2020年版[S]. 北京:中国医药科技出版社, 2020:211. [2] SUN P, ZHAO W J, WANG Q, et al. Chemical diversity, biological activities and traditional uses of and important Chinese herb Sophora[J]. Phytomedicine, 2022, 100:154054. doi: 10.1016/j.phymed.2022.154054 [3] SUN X Y, JIA L Y, RONG Z, et al. Research advances on matrine[J]. Front Chem, 2022, 10:867318. doi: 10.3389/fchem.2022.867318 [4] LONG G Q, WANG D D, WANG J, et al. Chemical constituents of Sophora flavescens Ait. and cytotoxic activities of two new compounds[J]. Nat Prod Res, 2022, 36(1):108-113. doi: 10.1080/14786419.2020.1765340 [5] HUAN D Q, HOP N Q, SON N T. Oxymatrine: a current overview of its health benefits[J]. Fitoterapia, 2023, 168:105565. doi: 10.1016/j.fitote.2023.105565 [6] 杨雪梅, 吴刚. 苦参碱抑制LPS诱导巨噬细胞IL-1β、TNF-α分泌及机制研究[J]. 中国免疫学杂志, 2016, 32(6):820-824,837. doi: 10.3969/j.issn.1000-484X.2016.06.011 [7] LI L X, NIU H, ZHAN J W, et al. Matrine attenuates bovine mammary epithelial cells inflammatory responses induced by Streptococcus agalactiae through inhibiting NF-κB and MAPK signaling pathways[J]. Int Immunopharmacol, 2022, 112:109206. doi: 10.1016/j.intimp.2022.109206 [8] LI J, CHENG X Y, YANG H, et al. Matrine ameliorates cognitive deficits via inhibition of microglia mediated neuroinflammation in an Alzheimer’s disease mouse model[J]. Pharmazie, 2020, 75(7):344-347. [9] ZHU T, ZHOU D, ZHANG Z, et al. Analgesic and antipruritic effects of oxymatrine sustained-release microgel cream in a mouse model of inflammatory itch and pain[J]. Eur J Pharm Sci, 2020, 141:105110. doi: 10.1016/j.ejps.2019.105110 [10] LI M X, YING M F, GU S L, et al. Matrine alleviates hypoxia-induced inflammation and pulmonary vascular remodelling via RPS5/NF-κB signalling pathway[J]. J Biochem Mol Toxicol, 2024, 38(1):e23583. doi: 10.1002/jbt.23583 [11] YING X J, JIN B, CHEN X W, et al. Oxymatrine downregulates HPV16E7 expression and inhibits cell proliferation in laryngeal squamous cell carcinoma Hep-2 cells in vitro[J]. Biomed Res Int, 2015, 2015:150390. [12] ZHUANG X Y, DONG A H, WANG R C, et al. Ursolic acid benzaldehyde chalcone, leads to inhibition of cell proliferation and arrests cycle in G1/G0 phase in colon cancer[J]. Saudi J Biol Sci, 2018, 25(8):1762-1766. doi: 10.1016/j.sjbs.2017.04.006 [13] ZHANG Y E, STUELTEN C H. Alternative splicing in EMT and TGF-β signaling during cancer progression[J]. Semin Cancer Biol, 2024, 101:1-11. doi: 10.1016/j.semcancer.2024.04.001 [14] CAO X J, HE Q Q. Anti-tumor activities of bioactive phytochemicals in Sophora flavescens for breast cancer[J]. Cancer Manag Res, 2020, 12:1457-1467. doi: 10.2147/CMAR.S243127 [15] HALIM C E, XINJING S L, FAN L, et al. Anti-cancer effects of oxymatrine are mediated through multiple molecular mechanism(s) in tumor models[J]. Pharmacol Res, 2019, 147:104327. doi: 10.1016/j.phrs.2019.104327 [16] RASOULI S, DAKIC A, WANG Q E, et al. Noncanonical functions of telomerase and telomeres in viruses-associated cancer[J]. J Med Virol, 2024, 96(5):e29665. doi: 10.1002/jmv.29665 [17] LI H J, WANG J M, TIAN Y T, et al. Effect of matrine on Fas, VEGF, and activities of telomerase of MCF-7 cells[J]. Chin J Integr Tradit West Med, 2013, 33(9):1247-1251. [18] LIU L, LIAO J Z, HE X X, et al. The role of autophagy in hepatocellular carcinoma: friend or foe[J]. Oncotarget, 2017, 8(34):57707-57722. doi: 10.18632/oncotarget.17202 [19] YU H P, ZHU B L, YANG W, et al. Matrine inhibits proliferation and migration of HepG2 cells by downregulating ERK1/2 signaling pathways[J]. J Cancer Res Ther, 2020, 16(2):209-214. doi: 10.4103/jcrt.JCRT_331_19 [20] LIANG L, WU J, LUO J, et al. Oxymatrine reverses 5-fluorouracil resistance by inhibition of colon cancer cell epithelial-mesenchymal transition and NF-κB signaling in vitro[J]. Oncol Lett, 2020, 19(1):519-526. [21] STRONATI G, GUERRA F, URBINATI A, et al. Tachycardiomyopathy in patients without underlying structural heart disease[J]. J Clin Med, 2019, 8(9):1411. doi: 10.3390/jcm8091411 [22] ZHANG X N, GAO Y Q, ZHOU Y T, et al. Pharmacological mechanism of natural drugs and their active ingredients in the treatment of arrhythmia via calcium channel regulation[J]. Biomedecine Pharmacother, 2023, 160:114413. doi: 10.1016/j.biopha.2023.114413 [23] MARUYAMA K, IMANAKA-YOSHIDA K. The pathogenesis of cardiac fibrosis: a review of recent progress[J]. Int J Mol Sci, 2022, 23(5):2617. doi: 10.3390/ijms23052617 [24] ZHANG X, HU C, ZHANG N, et al. Matrine attenuates pathological cardiac fibrosis via RPS5/p38 in mice[J]. Acta Pharmacol Sin, 2021, 42(4):573-584. doi: 10.1038/s41401-020-0473-8 [25] CAO Y G, JING S, LI L, et al. Antiarrhythmic effects and ionic mechanisms of oxymatrine from Sophora flavescens[J]. Phytother Res, 2010, 24(12):1844-1849. doi: 10.1002/ptr.3206 [26] WANG Z Y, ZU T H, HUANG X Z, et al. Comprehensive investigation of the content and the origin of matrine-type alkaloids in Chinese honeys[J]. Food Chem, 2023, 402:134254. doi: 10.1016/j.foodchem.2022.134254 [27] LIU W C, TIAN X, GU L W, et al. Oxymatrine mitigates Aspergillus fumigatus keratitis by suppressing fungal activity and restricting pyroptosis[J]. Exp Eye Res, 2024, 240:109830. doi: 10.1016/j.exer.2024.109830 [28] SUN T, LI X D, HONG J, et al. Inhibitory effect of two traditional Chinese medicine monomers, berberine and matrine, on the quorum sensing system of antimicrobial-resistant Escherichia coli[J]. Front Microbiol, 2019, 10:2584. doi: 10.3389/fmicb.2019.02584 [29] HARATA-LEE Y, QU Z P, BATEMAN E, et al. Compound Kushen injection reduces severity of radiation-induced gastrointestinal mucositis in rats[J]. Front Oncol, 2022, 12:929735. doi: 10.3389/fonc.2022.929735 [30] WANG W, YOU R L, QIN W J, et al. Anti-tumor activities of active ingredients in Compound Kushen Injection[J]. Acta Pharmacol Sin, 2015, 36(6):676-679. doi: 10.1038/aps.2015.24 [31] YANG Y, SUN M Y, LI W D, et al. Rebalancing TGF-β/Smad7 signaling via Compound kushen injection in hepatic stellate cells protects against liver fibrosis and hepatocarcinogenesis[J]. Clin Transl Med, 2021, 11(7):e410. doi: 10.1002/ctm2.410 [32] WU J Y, MA X Y, WANG X M, et al. Efficacy and safety of compound Kushen injection for treating advanced colorectal cancer: a protocol for a systematic review and meta-analysis[J]. Heliyon, 2024, 10(5):e26981. doi: 10.1016/j.heliyon.2024.e26981 [33] JIN Z S, HUANG Z H, WU C, et al. Molecular insights into gastric cancer: the impact of TGFBR2 and hsa-mir-107 revealed by microarray sequencing and bioinformatics[J]. Comput Biol Med, 2024, 172:108221. doi: 10.1016/j.compbiomed.2024.108221 [34] HUANG Z H, WU C, ZHOU W, et al. Compound kushen injection inhibits epithelial-mesenchymal transition of gastric carcinoma by regulating VCAM1 induced by the TNF signaling pathway[J]. Phytomedicine, 2023, 118:154984. doi: 10.1016/j.phymed.2023.154984 [35] LIU J, YU Q X, WANG X S, et al. Compound kushen injection reduces severe toxicity and symptom burden associated with curative radiotherapy in patients with lung cancer[J]. J Natl Compr Canc Ne, 2023, 21(8): 821-830(e3). [36] ZHAO Z Z, FAN H T, HIGGINS T, et al. Fufang Kushen injection inhibits sarcoma growth and tumor-induced hyperalgesia via TRPV1 signaling pathways[J]. Cancer Lett, 2014, 355(2):232-241. doi: 10.1016/j.canlet.2014.08.037 [37] LIU Y, HUANG J J, LI S C, et al. Advancements in hydrogel-based drug delivery systems for the treatment of inflammatory bowel disease: a review[J]. Biomater Sci, 2024, 12(4):837-862. doi: 10.1039/D3BM01645E [38] WANG X, CHEN W Y, JIN Y G, et al. Effect of Sophora flavescens alkaloid on aerobic vaginitis in gel form for local treatment[J]. Chung I Tsa Chih Ying Wen Pan, 2017, 37(3):314-320. [39] WANG N M, CUI L, MA C F, et al. Clinical observation on treatment of mycotic vaginitis with Sophora gel combined with Fluconazole capsules[J]. China J Chin Mater Med, 2015, 40(5):978-980. [40] 曾丽敏. 苦参凝胶治疗慢性宫颈炎的效果研究[J]. 世界复合医学, 2021, 7(12):155-158. [41] 檀龙海, 王俊霞, 黄晶, 等. 当归苦参丸联合克林霉素磷酸酯凝胶治疗玫瑰痤疮的临床研究[J]. 现代药物与临床, 2023, 38(7):1733-1736. [42] 陈爱东, 温超, 艾江, 等. 五味苦参胶囊联合水杨酸制剂治疗溃疡性结肠炎的效果及对IL-1β、Caspase-1、VACM-1水平的影响[J]. 中华养生保健, 2023, 41(16):30-33. [43] 陈奇. 复方苦参止痒软膏联合糠酸莫米松乳膏治疗慢性湿疹的临床效果[J]. 临床合理用药, 2023, 16(20):137-139,147. [44] LIN Y D, HE F M, WU L, et al. Matrine exerts pharmacological effects through multiple signaling pathways: a comprehensive review[J]. Drug Des Devel Ther, 2022, 16:533-569. doi: 10.2147/DDDT.S349678 [45] GU Y M, LU J Y, SUN W, et al. Oxymatrine and its metabolite matrine contribute to the hepatotoxicity induced by Radix Sophorae tonkinensis in mice[J]. Exp Ther Med, 2019, 17(4):2519-2528. [46] LU Z G, LI M H, WANG J S, et al. Developmental toxicity and neurotoxicity of two matrine-type alkaloids, matrine and sophocarpine, in zebrafish (Danio rerio) embryos/larvae[J]. Reprod Toxicol, 2014, 47:33-41. doi: 10.1016/j.reprotox.2014.05.015 [47] XU J, WANG K Q, XU W H, et al. The matrine derivate MASM prolongs survival, attenuates inflammation, and reduces organ injury in murine established lethal sepsis[J]. J Infect Dis, 2016, 214(11):1762-1772. doi: 10.1093/infdis/jiw445 [48] SUN B, XU M. Matrine inhibits the migratory and invasive properties of nasopharyngeal carcinoma cells[J]. Mol Med Rep, 2015, 11(6):4158-4164. doi: 10.3892/mmr.2015.3276 [49] LI L Y, LI J R, MA L Y, et al. SAR-guided development of indole-matrine hybrids as potential anticancer agents via mitochondrial stress/cytochrome c/caspase 3 signaling pathway[J]. Bioorg Chem, 2023, 134:106341. doi: 10.1016/j.bioorg.2023.106341 [50] WU L C, LIU S B, WEI J R, et al. Synthesis and biological evaluation of matrine derivatives as anti-hepatocellular cancer agents[J]. Bioorg Med Chem Lett, 2016, 26(17):4267-4271. doi: 10.1016/j.bmcl.2016.07.045 [51] NI W J, WANG L Z, SONG H J, et al. Synthesis and evaluation of 11-butyl matrine derivatives as potential anti-virus agents[J]. Molecules, 2022, 27(21):7563. doi: 10.3390/molecules27217563 [52] HU H G, WANG S Z, ZHANG C M, et al. Synthesis and in vitro inhibitory activity of matrine derivatives towards pro-inflammatory cytokines[J]. Bioorg Med Chem Lett, 2010, 20(24):7537-7539. doi: 10.1016/j.bmcl.2010.09.075 [53] XU W H, HU H G, TIAN Y, et al. Bioactive compound reveals a novel function for ribosomal protein S5 in hepatic stellate cell activation and hepatic fibrosis[J]. Hepatology, 2014, 60(2):648-660. doi: 10.1002/hep.27138 [54] FAN Z Y, CHEN Y P, CHEN L, et al. The matrine derivate MASM inhibits astrocyte reactivity and alleviates experimental autoimmune encephalomyelitis in mice[J]. Int Immunopharmacol, 2022, 108:108771. doi: 10.1016/j.intimp.2022.108771 [55] LI L Y, LU F F, DING S Q, et al. Pharmacokinetic, tissue distribution, metabolite, and toxicity evaluation of the matrine derivative, (6aS, 10S, 11aR, 11bR, 11cS)-10-methylaminododecahydro-3a, 7a-Diaza-benzo (de) anthracene-8-thione[J]. Molecules, 2024, 29(2):297. doi: 10.3390/molecules29020297 [56] LIU Y, QI Y, BAI Z H, et al. A novel matrine derivate inhibits differentiated human hepatoma cells and hepatic cancer stem-like cells by suppressing PI3K/AKT signaling pathways[J]. Acta Pharmacol Sin, 2017, 38(1):120-132. doi: 10.1038/aps.2016.104 -




 下载:
下载: