-
钙化性主动脉瓣疾病(CAVD),在老年人群体中较为常见,尤其是65岁及以上的患者中,发病率可达25~30%[1]。在瓣膜病变早期,脂质沉积、机械损伤及炎症反应等多种致病因素导致细胞内过氧化氢和超氧化物的过度积聚,引发DNA损伤,继而引起DNA修复机制功能失调,并通过上调AKT信号通路导致Runx2的表达,促进早期瓣膜间质细胞(VICs)表型改变[2-4]。虾青素(AST)是一种类胡萝卜素化合物,具有多种生物活性,包括抗氧化、抗炎和抗衰老等生物学功能[5]。有研究发现虾青素通过激活Nrf-2/HO-1信号通路抑制脊椎软骨终板中的氧化应激和钙化[6]。目前,虾青素对主动脉瓣钙化的影响鲜有报道。因此,本研究旨在探讨虾青素对主动脉瓣钙化的作用及其可能机制。
-
选取4月龄家猪(上海五丰食品有限公司),体质量约50 kg,获取主动脉瓣组织后,放入含1%青-链霉素的预冷无菌磷酸盐缓冲液中,置于冰盒迅速返回实验室进行细胞的分离与培养。
-
AST购自MedChemExpress公司;DMEM培养基、胎牛血清购自Gibco公司;CCK-8、活性氧检测试剂盒、茜素红染色液购自上海碧云天生物科技有限公司;精蛋白重组人胰岛素注射液为丹麦诺和诺德公司;二水合磷酸二氢钠购自国药集团化学试剂公司;L-抗坏血酸购自德国默克生命科学公司;CD31抗体、Vimentin抗体、Runx2抗体、Nrf2抗体购自武汉三鹰生物科技有限公司;ALP抗体购自江苏亲科生物有限公司;HO-1抗体购自英国Abcam公司。
-
用无菌PBS溶液冲洗掉瓣叶表面血液,加入Ⅰ型胶原酶,37 ℃摇床内消化15 min。后用无菌棉签擦去表面残留内皮细胞,无菌PBS溶液冲洗三次。再次加入4 mlⅠ型胶原酶,37 ℃摇床内消化5~8 h。200目一次性无菌滤网过滤后离心,使用15% FBS DMEM培养基重悬后铺板。2~3 d换液,传代至3~5代进行钙化诱导。成骨诱导培养基(OM)配方为:10−7mol/L胰岛素,50 μg/ml抗坏血酸,2 mmol/L磷酸二氢钠,5%胎牛血清,1%青霉素-链霉素的高糖DMEM培养基。
-
将VICs以2×104/孔接种到96孔板中。待细胞汇合度达50%时,加入不同浓度的AST溶液(0、5、10、20、50、100、200、400 μmol/L),继续培养过夜。根据CCK-8试剂盒说明书进行细胞生长活力检测。
-
OM培养7 d后,用无菌PBS清洗3次,用75%酒精固定10 min,吸去后再用无水乙醇脱水,吸去无水乙醇晾干见培养皿底变为干燥白色后加入500 μl茜素红染液,染色2 min后使用无水乙醇清洗至洗液变透明,进行镜下拍照。
-
提取细胞总蛋白后,使用BCA法测定蛋白浓度,然后进行十二烷基硫酸钠-聚丙烯酰胺凝胶电泳。转膜后在使用脱脂奶粉溶液室温下进行封闭1 h,TBST洗脱3次,每次5 min;一抗4 ℃孵育过夜(抗体稀释比例:Runx2 1∶
1000 ;ALP 1∶500;HO-1 1∶2000;Nrf2 1∶1000 );TBST洗脱3次后二抗室温孵育60 min;TBST洗脱3次,ECL显色液显影,凝胶成像系统显影,独立重复实验3次,Image J软件分析。 -
干预结束后PBS洗涤3次,每孔加入4%多聚甲醛室温固定细胞30 min;吸弃甲醛,PBS每3 min洗涤3次,用0.2% Triton X溶液室温破膜细胞20 min;PBS洗涤3次后每孔加入5% BSA封闭液,室温封闭1 h;吸弃封闭液,加入一抗(抗体稀释比例均为1:200)4 ℃过夜孵育;吸弃一抗,PBS洗涤3次后,加入二抗,室温避光孵育1 h;吸弃二抗标记后的抗体,加入PBS,避光条件下洗涤3次,最后加入含DAPI的抗荧光淬灭染剂,荧光显微镜下观察并拍照。
-
处理后的VICs用含DCFH-DA的培养液替代培养液,在37 ℃下孵育20 min。随后,使用荧光显微镜采集图像,并通过Image J软件分析图像的荧光强度。
-
所有实验数据采用“平均值±标准差”表示,应用GraphPad Prism 10.1.2软件进行统计分析。两组数据间比较采用t检验,多组数据间比较采用One-Way ANOVA及Post Hoc检验。P<0.05认为差异有统计学意义。
-
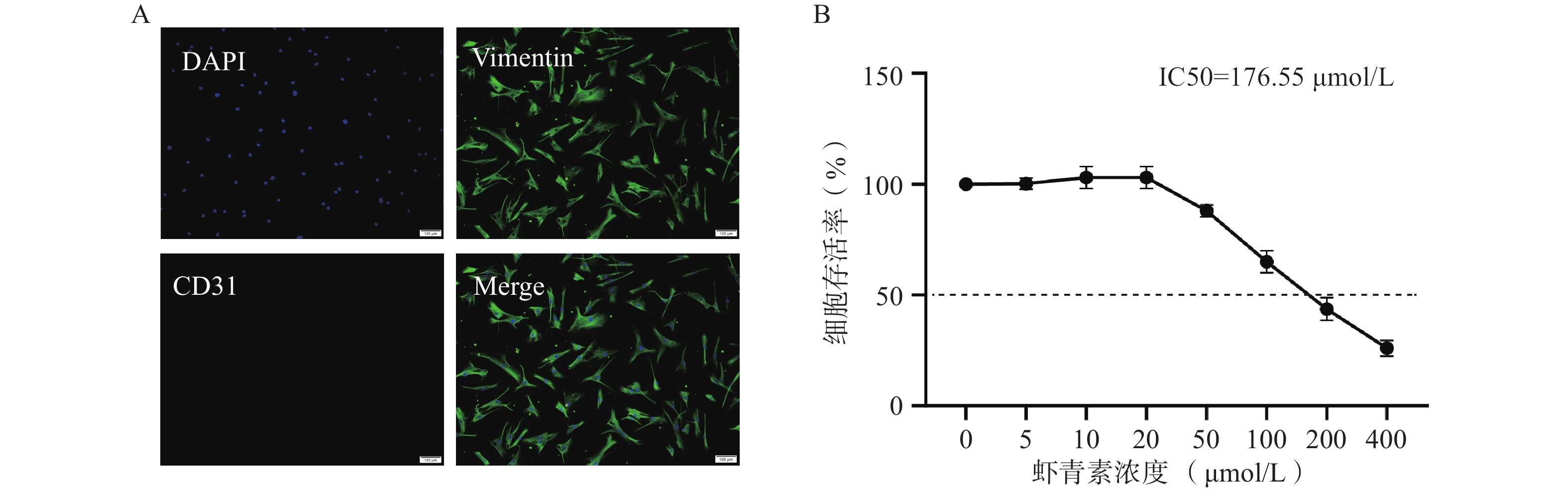
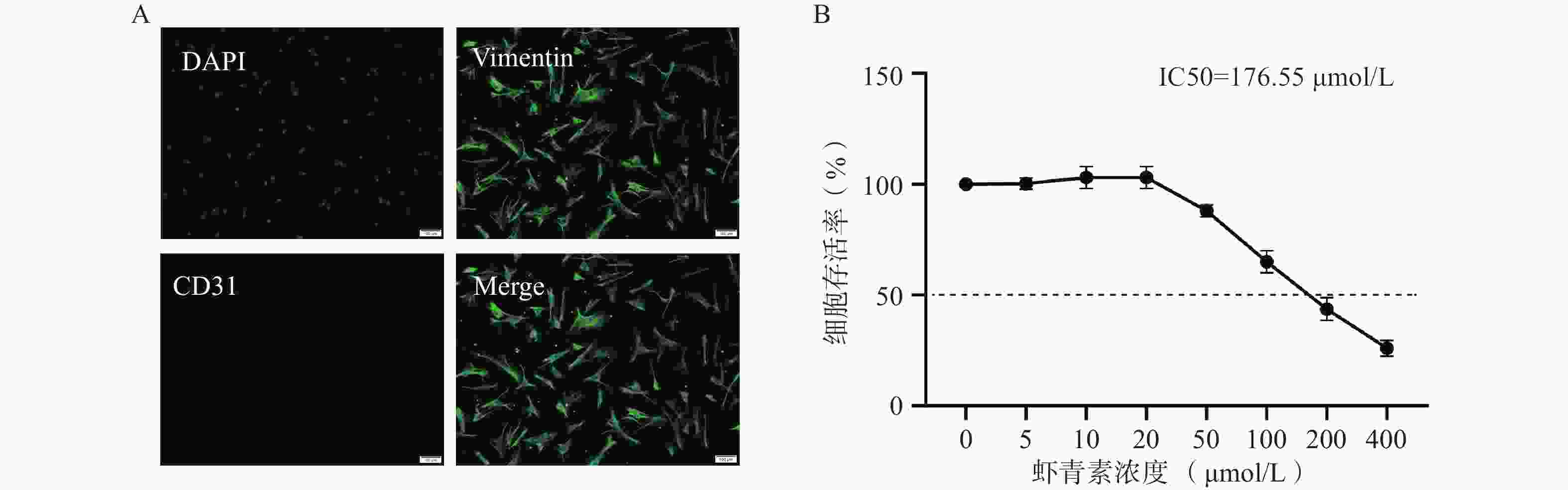
免疫荧光鉴定结果显示原代分离的猪VICs呈明显的间质细胞标志物Vimentin阳性,内皮标志物CD31阴性(图1A),细胞纯度超过95%,满足实验所需。CCK-8结果显示,浓度低于20 μmol/L时,AST处理不影响VICs的生长活力;AST浓度在50~400 μmol/L时,VICs的生长活力随AST浓度的增加而明显抑制(图1B)。后续实验采用浓度为20 μmol/L的AST作为干预浓度。
-
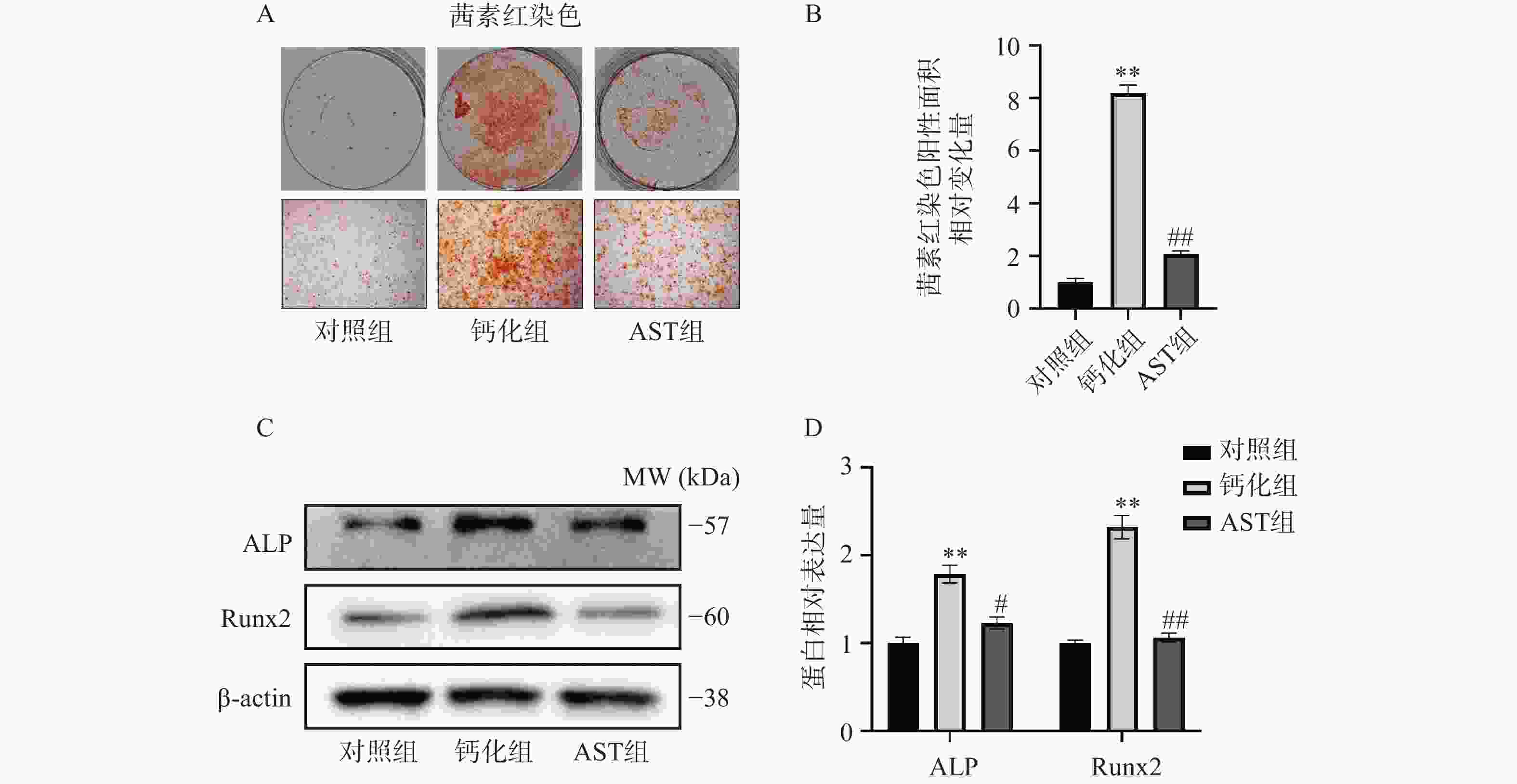
经OM成骨诱导后,茜素红染色后显示VICs形成明显的钙化结节,而AST处理的VICs钙化结节显著减少(图2A、2B)。Western blot检测结果显示,经OM成骨诱导后,VICs中成骨标志物ALP和Runx2的蛋白表达明显增加,而AST处理的VICs中两者表达的增加得到明显缓解(图2C、2D)。
-
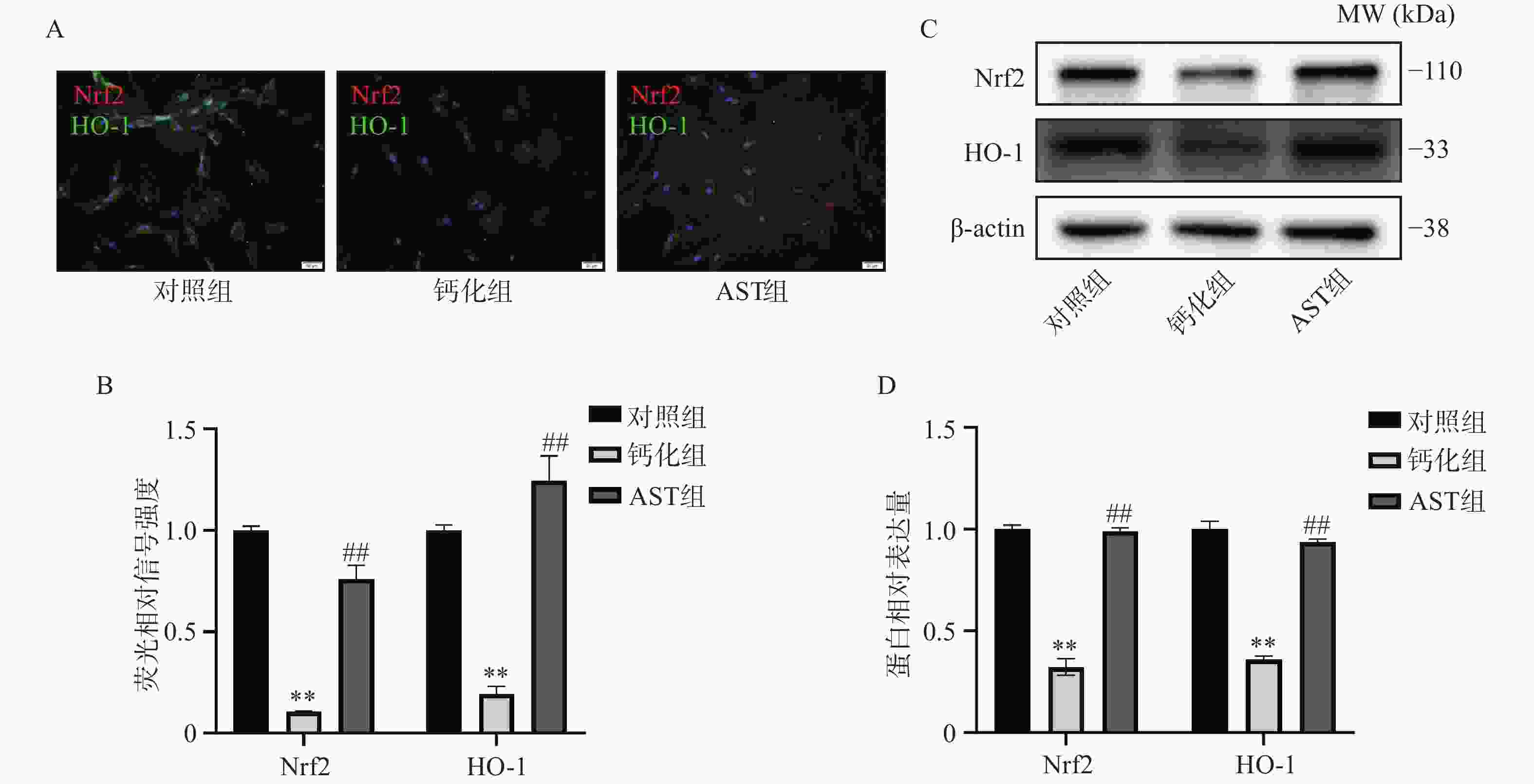
免疫荧光结果显示,Nrf2和HO-1的表达在OM诱导的VICs中明显降低;AST处理显著增加了两者的表达,且Nrf2的细胞定位主要集中在细胞核内(图3A-3B)。Western blot结果进一步证实,AST的处理显著缓解了OM诱导后Nrf2和HO-1表达的降低(图3C-3D)。
-
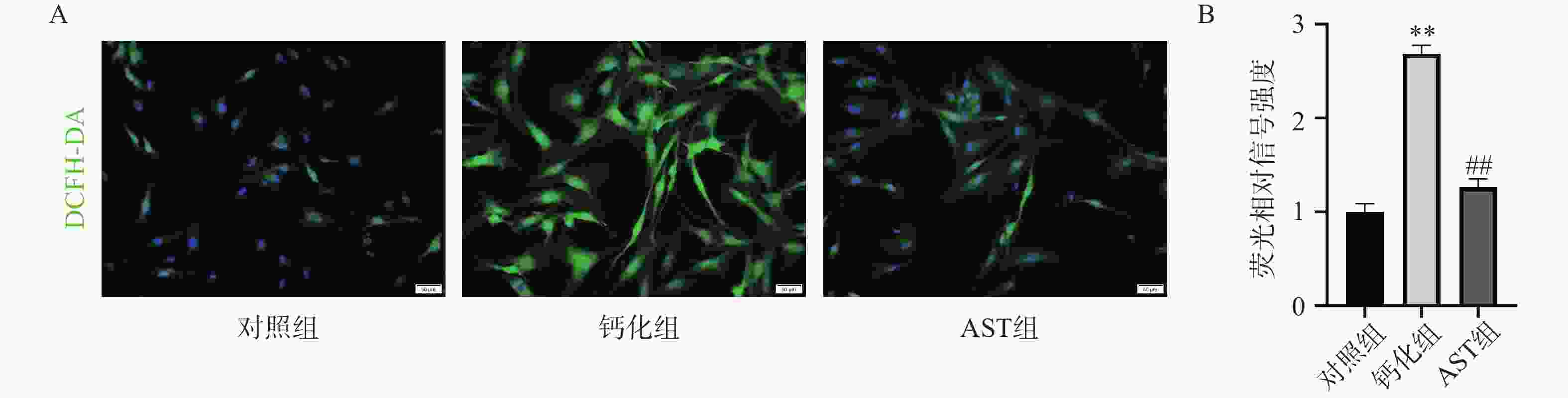
DCFH-DA染色结果显示,正常状态下VICs中ROS水平较低;OM诱导后,VICs中的ROS水平明显增加;AST处理后VICs中的ROS水平明显降低,为OM组的(47.09±5.46)%(图4A-4B)。
-
随着我国人口老龄化程度逐渐加重,CAVD已成为社会的严重负担,显著影响老年人的心脏健康[7-9]。寻找能够延缓或防止CAVD进展的新治疗策略具有重要意义。CAVD的发病机制复杂,涉及炎症反应、氧化应激、免疫、细胞外基质重构等多种生物学过程[10,11]。瓣膜间质细胞在各种病理因素作用下向成骨细胞转变,导致钙盐沉积及钙化灶形成[12,13]。
氧化应激(OS)是指体内或者细胞内氧自由基的生成与清除紊乱,导致活性氧化因子(ROS)异常积累,在体内或者细胞内积蓄从而引发氧化损伤的过程[14,15]。ROS通过氧化脂质、蛋白质和DNA,导致细胞功能障碍和凋亡,促进瓣膜纤维化和钙化。ROS激活NF-κB等炎症通路,诱导促炎因子(如IL-6、TNF-α)的表达,进一步加剧瓣膜损伤。然而,CAVD的发病机制相当复杂,确切机制尚未完全阐明。本研究发现,AST在体外钙化模型中能减轻瓣膜间质细胞成骨分化。从机制上看,AST通过增强Nrf2/HO-1抗氧化信号通路来抑制瓣膜间质细胞的钙化,突显了其作为CAVD新型治疗药物的潜力。这些发现表明,AST的抗氧化特性对其保护主动脉瓣的效果具有一定作用。
AST具有显著抗炎和抗氧化作用,被称为“超级抗氧化剂”[16,17]。在心血管、神经、肿瘤和骨关节炎领域的多项研究表明,AST可以通过激活Nrf2通路调节氧化应激,减少炎症反应并恢复线粒体功能[18,19]。炎症反应、氧化应激、机械应力、脂质代谢紊乱、年龄和其他因素可能会导致CAVD。在这些因素的影响下,瓣膜间质细胞发生病理变化,导致瓣叶钙化[20-22]。在过去的二十年中,越来越多的研究表明,ROS在CAVD中发挥重要作用[23,24]。ROS通过诱导氧化应激、炎症和钙化促进CAVD的发展,而Nrf2/HO-1通路通过抗氧化、抗炎和抗钙化作用发挥保护效应。在CAVD中,Nrf2/HO-1通路的失调可能导致氧化应激加剧和瓣膜病变进展。我们的体外结果表明,AST激活了VICs的Nrf2/HO-1信号通路,有效减少了VICs中的ROS生成,维持氧化还原稳态最终抑制钙化。
本研究发现AST在抑制主动脉瓣钙化方面显示出较好的结果,但该实验仅在体外进行,后续仍需在大规模临床试验中进一步验证其长期安全性和有效性。研究还应考虑AST与其他药物之间的相互作用,以确保最佳的应用适应症。此外,AST还具有其他可能抑制钙化的作用,如减轻炎症反应等。总的来说,AST展现出抑制主动脉瓣钙化的潜力,其在心血管疾病预防和治疗中的应用值得进一步研究。未来的研究将有助于明确AST提升心血管健康的具体机制及临床意义。
The inhibition effect of astaxanthin on calcification of aortic valve interstitial cells by activating the Nrf2/HO-1 Pathway
-
摘要:
目的 研究虾青素(AST)对成骨诱导培养基(OM)诱导瓣膜间质细胞(VICs)钙化作用和机制。 方法 采用CCK-8分析不同浓度虾青素对VICs增殖的影响;虾青素干预OM培养VICs后茜素红染色检测钙结节,Western Blot检测成骨分化标志ALP、Runx2蛋白表达水平;Western Blot及细胞免疫荧光检测细胞Nrf2、HO-1蛋白表达水平;DCFH-DA荧光染色检测细胞内活性氧(ROS)水平。 结果 CCK-8结果显示虾青素适宜干预浓度为25 μmol/L;虾青素处理减轻OM诱导VICs钙结节形成,抑制VICs的成骨分化标志ALP、Runx2的表达(P<0.01),增强Nrf2和HO-1的表达,并降低了细胞内活性氧的水平(P<0.01)。 结论: 虾青素可能通过增强Nrf2/HO-1抗氧化信号通路减轻VICs氧化应激水平与钙化。 -
关键词:
- 虾青素 /
- 钙化 /
- 瓣膜间质细胞 /
- 氧化应激 /
- Nrf2/HO-1通路
Abstract:Objective To clarify the effect and mechanisms of astaxanthin on the calcification of aortic valve interstitial cells (VICs) induced by osteogenic medium (OM). Methods The CCK-8 assay was used to analyze the effects of different concentrations of astaxanthin on the proliferation of VICs. After treating VICs with astaxanthin in OM, Alizarin Red staining was performed to detect calcified nodules, and Western blotting was used to measure the expression levels of osteogenic differentiation markers ALP and Runx2. Additionally, Western blotting and immunofluorescence detection were utilized to assess the expression levels of Nrf2 and HO-1 proteins. The levels of intracellular reactive oxygen species (ROS) were measured by DCFH-DA fluorescence staining. Results The CCK-8 results indicated that the optimal concentration of astaxanthin for intervention was 25 μmol/L. Astaxanthin treatment reduced the formation of calcified nodules induced by OM in VICs, and inhibited the expression of osteogenic differentiation markers ALP and Runx2 (P<0.01). Furthermore, Astaxanthin treatment decreased the expression levels of Nrf2 and HO-1 (P<0.01), and reduced intracellular reactive oxygen species levels (P<0.01). Conclusion Astaxanthin may mitigate oxidative stress and calcification in VICs by enhancing the Nrf2/HO-1 antioxidant signaling pathway. -
Key words:
- Astaxanthin /
- Calcification /
- Valve interstitial cells /
- Oxidative stress /
- Nrf2/HO-1 Pathway
-
-
[1] GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2018, 392(10159):1789-1858. [2] BRANCHETTI E, SAINGER R, POGGIO P, et al. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis[J]. Arterioscler Thromb Vasc Biol, 2013, 33(2):e66-74. [3] LIBERMAN M, BASSI E, MARTINATTI M K, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification[J]. Arterioscler Thromb Vasc Biol, 2008, 28(3):463-470. doi: 10.1161/ATVBAHA.107.156745 [4] SHU L, YUAN Z, LI F, et al. Oxidative stress and valvular endothelial cells in aortic valve calcification[J]. Biomed Pharmacother, 2023, 163:114775. [5] LI Y F, LI J F, XU S, et al. Tetrahedral framework nucleic acid-based delivery of astaxanthin suppresses chondrocyte pyroptosis and modulates oxidative stress for the treatment of osteoarthritis[J]. Adv Healthc Mater, 2024, 13(28):e2401452. [6] YANG G H, LIU X Y, JING X Z, et al. Astaxanthin suppresses oxidative stress and calcification in vertebral cartilage endplate via activating Nrf-2/HO-1 signaling pathway[J]. Int Immunopharmacol, 2023, 119:110159. [7] MARTIN S S, ADAY A W, ALMARZOOQ Z I, et al. 2024 heart disease and stroke statistics: a report of US and global data from the American heart association[J]. Circulation, 2024, 149(8):e347-e913. [8] CONRAD N, MOLENBERGHS G, VERBEKE G, et al. Trends in cardiovascular disease incidence among 22 million people in the UK over 20 years: population based study[J]. BMJ, 2024, 385:e078523. [9] STRANGE G, STEWART S, CELERMAJER D, et al. Poor long-term survival in patients with moderate aortic stenosis[J]. J Am Coll Cardiol, 2019, 74(15):1851-1863. [10] MONCLA L M, BRIEND M, BOSSÉ Y, et al. Calcific aortic valve disease: mechanisms, prevention and treatment[J]. Nat Rev Cardiol, 2023, 20(8):546-559. [11] PARRA-IZQUIERDO I, SÁNCHEZ-BAYUELA T, LÓPEZ J, et al. Interferons are pro-inflammatory cytokines in sheared-stressed human aortic valve endothelial cells[J]. Int J Mol Sci, 2021, 22(19):10605. doi: 10.3390/ijms221910605 [12] IQBAL F, SCHLOTTER F, BECKER-GREENE D, et al. Sortilin enhances fibrosis and calcification in aortic valve disease by inducing interstitial cell heterogeneity[J]. Eur Heart J, 2023, 44(10):885-898. [13] ZHENG K H, TZOLOS E, DWECK M R. Pathophysiology of aortic stenosis and future perspectives for medical therapy[J]. Cardiol Clin, 2020, 38(1):1-12. [14] SIES H, JONES D P. Reactive oxygen species(ROS)as pleiotropic physiological signalling agents[J]. Nat Rev Mol Cell Biol, 2020, 21(7):363-383. doi: 10.1038/s41580-020-0230-3 [15] FORRESTER S J, KIKUCHI D S, HERNANDES M S, et al. Reactive oxygen species in metabolic and inflammatory signaling[J]. Circ Res, 2018, 122(6):877-902. doi: 10.1161/CIRCRESAHA.117.311401 [16] JIANG X D, ZU L, WANG Z Y, et al. Micro-algal astaxanthin could improve the antioxidant capability, immunity and ammonia resistance of juvenile Chinese mitten crab, Eriocheir sinensis[J]. Fish Shellfish Immunol, 2020, 102:499-510. [17] ZHANG Y, YE Y, DING W, et al. Astaxanthin is ketolated from Zeaxanthin independent of fatty acid synthesis in Chromochloris zofingiensis[J]. Plant Physiol, 2020, 183(3):883-897. [18] PEREIRA C P M, SOUZA A C R, VASCONCELOS A R, et al. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (Review)[J]. Int J Mol Med, 2021, 47(1):37-48. [19] SONG X D, WANG B S, LIN S C, et al. Astaxanthin inhibits apoptosis in alveolar epithelial cells type II in vivo and in vitro through the ROS-dependent mitochondrial signalling pathway[J]. J Cell Mol Med, 2014, 18(11):2198-2212. doi: 10.1111/jcmm.12347 [20] XIE K J, ZENG J X, WEN L M, et al. Abnormally elevated EZH2-mediated H3K27me3 enhances osteogenesis in aortic valve interstitial cells by inhibiting SOCS3 expression[J]. Atherosclerosis, 2023, 364:1-9. doi: 10.1016/j.atherosclerosis.2022.11.017 [21] BOSSE K, HANS C P, ZHAO N, et al. Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease[J]. J Mol Cell Cardiol, 2013, 60:27-35. [22] LIU Z T, WANG K, JIANG C, et al. Morusin alleviates aortic valve calcification by inhibiting valve interstitial cell senescence through Ccnd1/Trim25/Nrf2 axis[J]. Adv Sci, 2024, 11(20):e2307319. doi: 10.1002/advs.202307319 [23] LI J Y, ZENG Q C, XIONG Z Y, et al. Trimethylamine N-oxide induces osteogenic responses in human aortic valve interstitial cells in vitro and aggravates aortic valve lesions in mice[J]. Cardiovasc Res, 2022, 118(8):2018-2030. [24] PENG X, SU S W, ZENG J X, et al. 4-Octyl itaconate suppresses the osteogenic response in aortic valvular interstitial cells via the Nrf2 pathway and alleviates aortic stenosis in mice with direct wire injury[J]. Free Radic Biol Med, 2022, 188:404-418. doi: 10.1016/j.freeradbiomed.2022.06.246 -




 下载:
下载: