-
本课题组通过小鼠胚胎干细胞(ES细胞)基因打靶,获得了ZF-12+/-(又称Zfp191,是ZNF24的同源基因)ES细胞,经囊胚腔显微注射后获得的ZF-12+/-小鼠生长发育正常,但ZF-12-/-小鼠胚胎发育明显滞后,且在7.5 d死亡[3]。ZNF24在小鼠胚胎发育过程中的表达谱为:E6.5~E7.5时 ZNF24在外胚层和中胚层中普遍表达;E8.5时ZNF24在神经外胚层中普遍表达;从E9.5开始,ZNF24在外胚层、内胚层和中胚层的几个组织中都普遍表达[4]。这些研究提示ZNF24在胚胎的发育过程中可能起重要作用。
-
小鼠胚胎、成年大鼠脑室周围和脊髓等中枢神经细胞增殖活跃的区域中ZNF24 mRNA表达水平较高,但中枢神经细胞向边缘移行的区域中ZNF24 mRNA表达水平较低[4]。在人神经祖细胞中,通过siRNA干扰下调ZNF24表达水平,细胞增殖受抑制,并进入分化状态;而过表达上调ZNF24表达水平,祖细胞分化受阻滞,细胞S期增加,促进细胞增殖[4-5]。最近研究表明,在血管内动脉损伤中,ZNF24表达下调可抑制血管平滑肌细胞增殖与迁移,进而抑制血管内膜增生[6];在内皮细胞的血管生成中,ZNF24可调控微血管内皮细胞的增殖、迁移和侵袭[7-8];ZNF24对于少突胶质细胞的中枢神经系统髓鞘形成以及维持神经祖细胞分裂状态是必不可少的[5]。此外,本课题组通过基因过表达与siRNA干扰结合基因芯片技术分析表明,ZNF24参与激酶(如Protein kinase A、Protein kinase D3和Neuregulin 1等)转录活性调控、血管增生、脑发育以及DNA损伤应答等,表明ZNF24是一个多功能因子[9]。这些研究提示ZNF24在细胞增殖、分化、迁移和侵袭中起重要的作用。
-
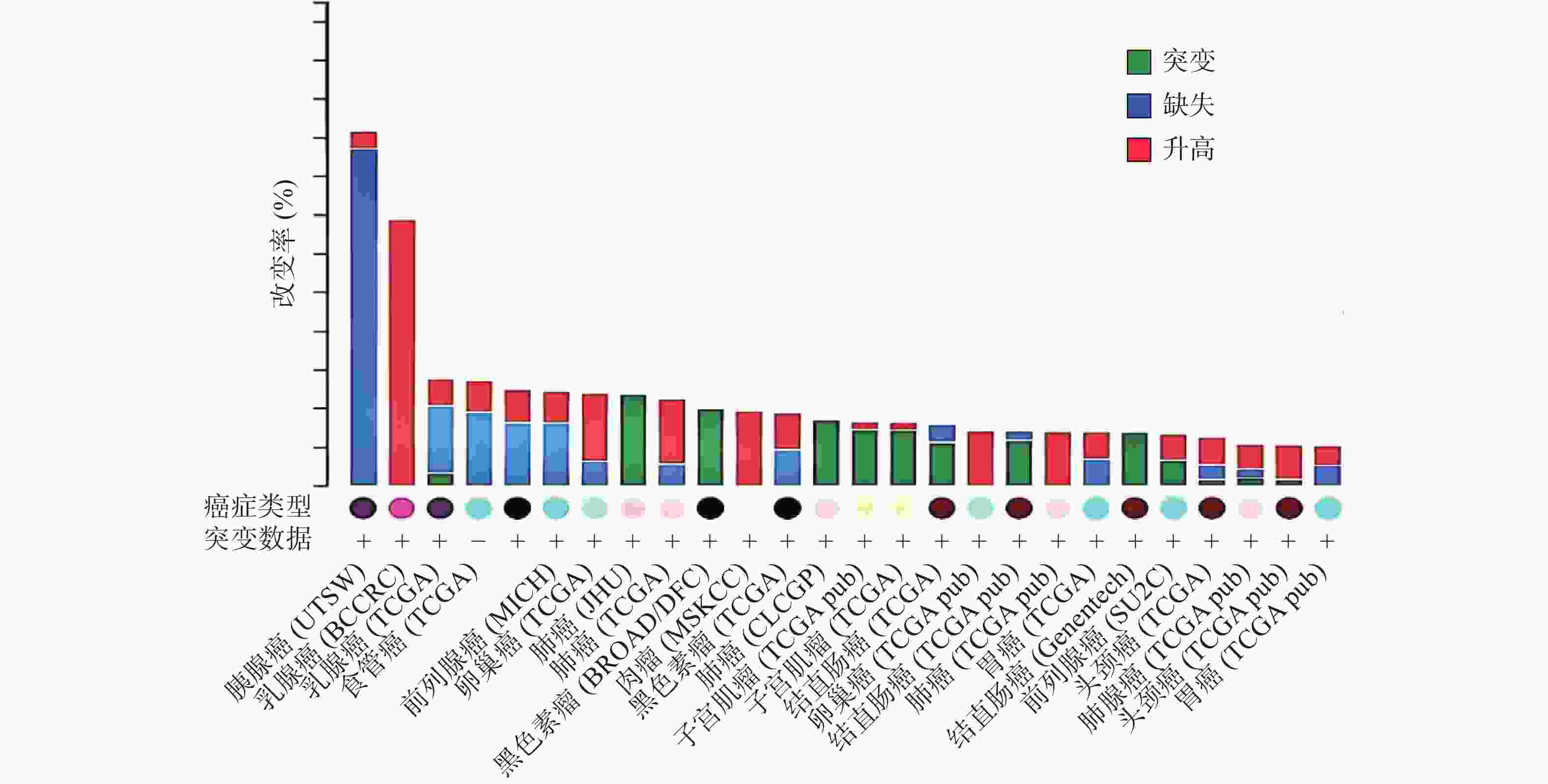
ZNF24基因定位于染色体18q 12.1[1-2, 10],该区域的缺失和突变常在人类多种恶性肿瘤中发现[11-15]。癌症基因组图谱(TCGA)收录了各种人类癌症的临床数据、基因组变异、mRNA表达、miRNA表达和甲基化等数据[16]。有研究[17]分析了 TCGA中ZNF24在各肿瘤中的变化情况,如图2所示,ZNF24在乳腺癌、卵巢癌、宫颈癌和膀胱癌中表达升高;在肺癌、黑色素瘤、结直肠癌和肝癌中发生突变;在胰腺癌、前列腺癌和食管癌中90%以上发生缺失,这些证据提示ZNF24与多种恶性肿瘤相关密切。下面将结合近年来的研究对ZNF24在恶性肿瘤中的作用及机制进行探讨。
-
前列腺癌(PCa)是男性泌尿生殖系统中最常见的癌症,2018年PCa成为第二大常见癌症,并且是男性癌症死亡的第五大原因[12]。PCa是一种异质性较大的肿瘤,易发生转移[18]。转移的初始环节是上皮-间质细胞转化(EMT)[19],其主要特征是上皮细胞的粘附能力丧失与间充质样形态的增加[20]。典型的EMT表型是E-cadherin的下调和N-cadherin的上调[21]。肿瘤细胞通过EMT增强其侵袭能力,导致肿瘤转移[22]。多研究表明EMT相关的转录因子可激活EMT,如Twist1 转录因子[23]。最近研究表明,ZNF24在前列腺癌中表达上调,可促进细胞增殖,并且通过调控Twist1,诱导EMT过程,促进细胞迁移和侵袭[24],提示ZNF24在前列腺癌发生、发展中是癌基因。
-
胃癌(GC)是全球第五大常见癌症,也是癌症相关死亡的第三大原因[12],目前治疗胃癌的主要和传统方法仍然是手术切除[11],然而大多数患者在切除后仍会复发,即使采用当下先进的系统治疗,5年总生存率也不超过20%[15]。因此,越来越多的人致力于研究胃癌进展的机制,特别是胃癌转移的机制。证据表明,microRNA(miRNA)在胃癌的进展和转移中起着重要作用。其中在研究microRNA-940(miRNA-940)在胃癌中的作用时,发现ZNF24是其调控的靶基因,即miRNA-940 可通过靶向抑制ZNF24表达,促进肿瘤细胞的侵袭和转移[14],提示ZNF24抑制胃癌细胞的侵袭和转移,表现为抑癌基因。
-
肝细胞癌(HCC)是一种原发性肝癌,是全球第四大常见癌症,也是癌症相关死亡的第二大原因,每年死亡人数高达78万人[25]。在肝细胞癌的发生发展过程中,有多种细胞信号转导通路被激活,如Wnt/β-catenin、p53、pRb、MAPK、Ras通路等,其中报道最多的是典型的Wnt/β-catenin信号通路[26-28]。
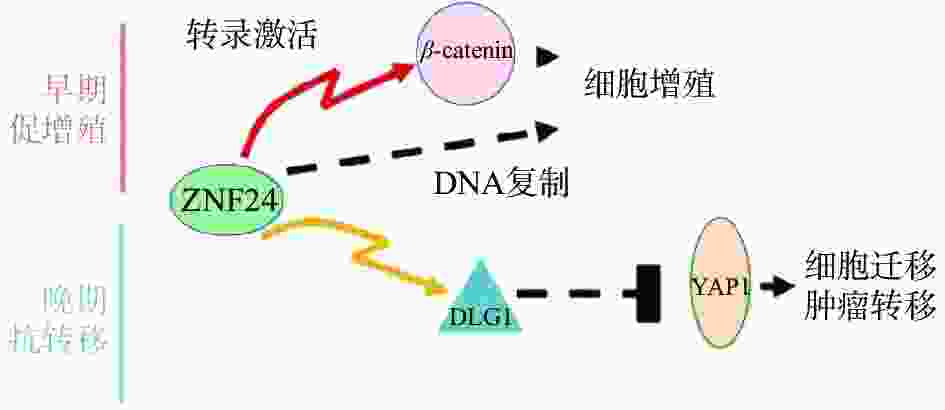
复旦大学余龙教授科研团队报道ZNF24在肝癌组织中表达上调,通过与β-连环蛋白(β-catenin)基因的启动子上ATTAATT序列特异结合,激活β-catenin基因转录,进而激活其下游靶基因如细胞周期蛋白 D1(Cyclin D1)基因,促进肝癌细胞的增殖[28]。另有报道ZNF24与Wnt8B(Wnt家族成员8B)的启动子上的ATTAATT和ATTCATT序列特异结合,转录激活Wnt8B表达,进而激活Wnt信号通路,促进肝癌细胞的细胞增殖,提示ZNF24在肝癌中是癌基因[29]。令人感兴趣的是,余龙教授团队发现在转移肝癌组织中ZNF24表达下调,ZNF24通过与DGL1(Discs Large 1)启动子上的(TCAT)n重复序列特异结合,激活DGL1基因转录,通过Yes相关蛋白(YAP)信号通路抑制肝癌细胞的转移,提示ZNF24在肝癌转移中是抑癌基因[30]。以上研究表明ZNF24在肝癌中的不同功能:在肝癌早期阶段ZNF24与β-catenin的启动子结合,并激活其转录,促进肝癌细胞增殖;在肝癌晚期阶段,ZNF24表达下调,进而DLG1表达水平下调,激活YAP1活性,最终导致肝癌细胞的转移(图3)[30]。
-
甲状腺癌(THCA)是内分泌系统常见的恶性肿瘤,其发病率约占所有恶性肿瘤的2.5%[31]。由于诊断技术、环境(如辐射、污染)和生活方式的改变,在过去10年里,THCA的发病率逐年增长[32],虽然包括免疫治疗在内的各种治疗方式都取得了进展,但由于在分子水平上对THCA发生机制的认识不足[33],导致患者的预后仍然不理想。ZNF24在THCA中表达下调,通过竞争性结合β-catenin,抑制β-catenin与辅助因子LEF1/TCF1形成功能性复合物,从而抑制Wnt信号通路,进而抑制肿瘤增生与转移[34],提示ZNF24在THCA的发生与发展中是抑癌基因。
-
乳腺癌是女性最常见的肿瘤[35],大约1/3的病例会发展成转移性疾病[36],是癌症相关死亡的主要原因[37]。更令人不安的是,即使成功切除原发性肿瘤,局部发生转移的风险仍然很高[38]。对人乳腺癌裸鼠移植瘤模型的研究表明,ZNF24通过与血管内皮生长因子(VEGF)启动子上游序列[-144/-134,非(TCAT)n重复序列] 直接结合抑制VEGF基因转录,阻断肿瘤新生血管的形成,从而抑制肿瘤细胞的生长,提示ZNF24是肿瘤新生血管的抑制剂[39]。值得一提的是,研究miRNA-940在肿瘤中的作用时,发现ZNF24是其调控的靶基因,即在三阴乳腺癌(TNBC)中miRNA-940可靶向下调ZNF24的表达,抑制TNBC细胞的增殖和转移[40],提示ZNF24促进TNBC细胞的增殖和转移,是癌基因。
-
ZNF24是一个多功能转录因子,参与激酶转录活性调控、血管增生、发育等,尤其在肿瘤发生或发展中起着重要的作用。ZNF24通过调控不同靶基因(如VEGF、Wnt8B、Twist1、β-catenin和DGL1等)的转录表达以及竞争性结合蛋白因子(如β-catenin),在肿瘤的发生、发展、侵袭和转移中起着重要复杂的两面性作用(促进和抑制)。因此,阐明其具体分子机制以及在肿瘤中的作用机理,将为肿瘤的治疗提供线索与思路。
Research progress on transcription factor ZNF24 in Tumors
-
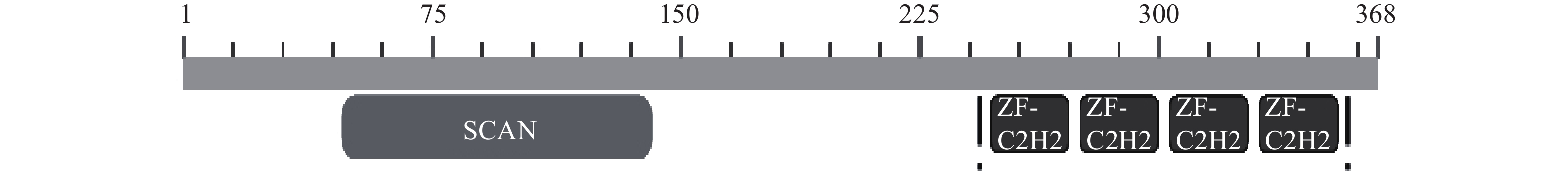
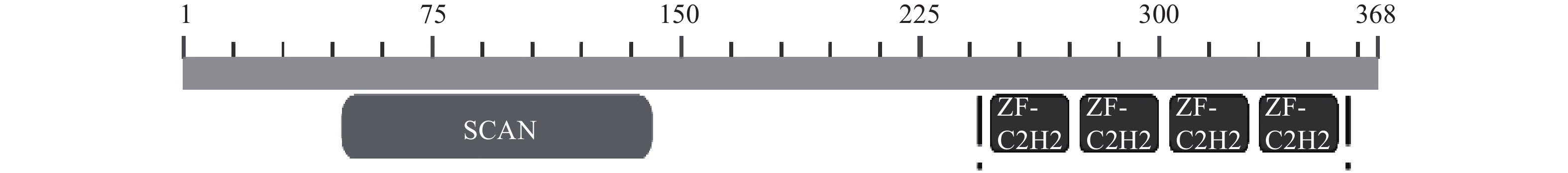
摘要: 转录因子 ZNF24(也称 ZNF191 或 KOX17)是类 Krüppel 锌指转录因子家族成员,N 端有富含亮氨酸(Leu)的 SCAN 结构域(又称 LeR 结构域),C 端有 4 个连续的典型的类 Krüppel样锌指模体。ZNF24参与激酶转录活性调控、血管增生、发育等,尤其在肿瘤发生或发展中起着重要的作用,是多功能的转录因子。ZNF24通过调控不同靶基因(如VEGF、Wnt8B、Twist1、β-catenin和DGL1等)的转录表达以及竞争性结合蛋白因子(如β-catenin),在肿瘤的发生、发展、侵袭和转移中起着重要复杂的两面性作用(促进和抑制)。因此阐明ZNF24在肿瘤中的作用机理,将为肿瘤的治疗提供线索与思路。本文将对ZNF24在肿瘤中的研究进展作一综述。Abstract: The transcription factor ZNF24 (also known as ZNF191 or KOX17) is a member of the Krüppel-like zinc finger transcription factor family, with a leucine-rich (Leu) SCAN domain (also known as LeR domain) at the N-terminus and four consecutive typical Kruppel-like zinc finger modities at the C-terminus. ZNF24 is a multifunctional transcription factor involved in the regulation of kinase transcriptional activity, vascular proliferation and development, especially in tumorigenesis and tumor progression. ZNF24 plays an important and complex dual-directional regulation role (promoting and inhibiting) in tumor development, invasion and metastasis by regulating the transcriptional expression of different target genes (such as VEGF, Wnt8B, Twist1, β-catenin and DGL1, etc.) and the competitive binding with protein factors (such as β-catenin). Therefore, elucidating the mechanism of ZNF24 in tumors would provide clues and ideas for the treatment of tumors. The researches status of ZNF24 in tumors were summarized in this paper.
-
Key words:
- ZNF 24 /
- tumor /
- transcription factors
-
[1] ROUSSEAU-MERCK M F, HUEBNER K, BERGER R, et al. Chromosomal localization of two human zinc finger protein genes, ZNF24 (KOX17) and ZNF29 (KOX26), to 18q12 and 17p13-p12, respectively[J]. Genomics,1991,9(1):154-161. doi: 10.1016/0888-7543(91)90233-5 [2] HAN Z G, ZHANG Q H, YE M, et al. Molecular cloning of six novel Krüppel-like zinc finger genes from hematopoietic cells and identification of a novel transregulatory domain KRNB[J]. J Biol Chem,1999,274(50):35741-35748. doi: 10.1074/jbc.274.50.35741 [3] LI J Z, CHEN X, YANG H, et al. The zinc finger transcription factor 191 is required for early embryonic development and cell proliferation[J]. Exp Cell Res,2006,312(20):3990-3998. doi: 10.1016/j.yexcr.2006.08.020 [4] KHALFALLAH O, FAUCON-BIGUET N, NARDELLI J, et al. Expression of the transcription factor Zfp191 during embryonic development in the mouse[J]. Gene Expr Patterns,2008,8(3):148-154. doi: 10.1016/j.gep.2007.11.002 [5] KHALFALLAH O, RAVASSARD P, LAGACHE C S, et al. Zinc finger protein 191 (ZNF191/Zfp191) is necessary to maintain neural cells as cycling progenitors[J]. Stem Cells,2009,27(7):1643-1653. doi: 10.1002/stem.88 [6] LV L, ZHANG J W, WANG P, et al. Zinc finger protein 191 deficiency attenuates vascular smooth muscle cell proliferation, migration, and intimal hyperplasia after endovascular arterial injury[J]. J Vasc Surg,2014,59(2):500-509. doi: 10.1016/j.jvs.2013.03.049 [7] JIA D, HUANG L, BISCHOFF J, et al. The endogenous zinc finger transcription factor, ZNF24, modulates the angiogenic potential of human microvascular endothelial cells[J]. FASEB J,2015,29(4):1371-1382. doi: 10.1096/fj.14-258947 [8] HUANG M T, HUANG X, JIANG B M, et al. linc00174-EZH2-ZNF24/runx1-VEGFA regulatory mechanism modulates post-burn wound healing[J]. Mol Ther Nucleic Acids,2020,21:824-836. doi: 10.1016/j.omtn.2020.07.010 [9] LI J Z, CHEN X, GONG X L, et al. A transcript profiling approach reveals the zinc finger transcription factor ZNF191 is a pleiotropic factor[J]. BMC Genomics,2009,10:241. doi: 10.1186/1471-2164-10-241 [10] SHI S L, LIU M M, YU L, et al. Assignment of a novel zinc finger gene ZNF191 to human chromosome 18Q12.1 by human/rodent somatic cell hybrid panel and fluorescent in situ hybridization[J]. Shi Yan Sheng Wu Xue Bao,1998,31(1):21-27. [11] AURELLO P, MINERVINI A, PACE M, et al. The role of surgery in the treatment of metachronous liver metastasis from gastric cancer: a systematic review[J]. Anticancer Res,2022,42(1):25-33. doi: 10.21873/anticanres.15453 [12] SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2021,71(3):209-249. doi: 10.3322/caac.21660 [13] LI H X, LI Y, TIAN D M, et al. miR-940 is a new biomarker with tumor diagnostic and prognostic value[J]. Mol Ther Nucleic Acids,2021,25:53-66. doi: 10.1016/j.omtn.2021.05.003 [14] LIU X Y, GE X X, ZHANG Z, et al. microRNA-940 promotes tumor cell invasion and metastasis by downregulating ZNF24 in gastric cancer[J]. Oncotarget,2015,6(28):25418-25428. doi: 10.18632/oncotarget.4456 [15] WAGNER A D, GROTHE W, HAERTING J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data[J]. J Clin Oncol,2006,24(18):2903-2909. doi: 10.1200/JCO.2005.05.0245 [16] WEINSTEIN J N, COLLISSON E A, MILLS G B, et al. The Cancer Genome Atlas Pan-Cancer analysis project[J]. Nat Genet,2013,45(10):1113-1120. doi: 10.1038/ng.2764 [17] HOU L M, CHEN M S, YANG H W, et al. MiR-940 Inhibited Cell Growth and Migration in Triple-Negative Breast Cancer[J]. Medical science monitor:international medical journal of experimental and clinical research,2016,22:3666-3672. [18] WANG Z P, ZHU S, ZHAO J G, et al. The heterogeneity of intraductal carcinoma of the prostate is associated with different efficacy of standard first-line therapy for patients with metastatic castration-resistant prostate cancer[J]. Prostate,2021,81(15):1191-1201. doi: 10.1002/pros.24215 [19] SAVAGNER P. Epithelial-mesenchymal transitions: from cell plasticity to concept elasticity[J]. Curr Top Dev Biol,2015,112:273-300. [20] DAS V, Bhattacharya S, Chikkaputtaiah C, et al. The basics of epithelial-mesenchymal transition(EMT): a study from a structure, dynamics, and functional perspective[J]. J Cell Physiol,2019:2019Feb5. [21] ODERO-MARAH V, HAWSAWI O, HENDERSON V, et al. Epithelial-mesenchymal transition (EMT) and prostate cancer[J]. Adv Exp Med Biol,2018,1095:101-110. [22] YAO D B, DAI C L, PENG S L. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation[J]. Mol Cancer Res,2011,9(12):1608-1620. doi: 10.1158/1541-7786.MCR-10-0568 [23] WANG X H, LING M T, GUAN X Y, et al. Identification of a novel function of TWIST, a bHLH protein, in the development of acquired taxol resistance in human cancer cells[J]. Oncogene,2004,23(2):474-482. doi: 10.1038/sj.onc.1207128 [24] HUANG X J, LIU N X, XIONG X. ZNF24 is upregulated in prostate cancer and facilitates the epithelial-to-mesenchymal transition through the regulation of Twist1[J]. Oncol Lett,2020,19(5):3593-3601. [25] FERLAY J, COLOMBET M, SOERJOMATARAM I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods[J]. Int J Cancer,2019,144(8):1941-1953. doi: 10.1002/ijc.31937 [26] ALQAHTANI A, KHAN Z, ALLOGHBI A, et al. Hepatocellular carcinoma: molecular mechanisms and targeted therapies[J]. Medicina (Kaunas),2019,55(9):526. doi: 10.3390/medicina55090526 [27] LACHENMAYER A, ALSINET C, SAVIC R, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib[J]. Clin Cancer Res,2012,18(18):4997-5007. doi: 10.1158/1078-0432.CCR-11-2322 [28] LIU G, JIANG S, WANG C, et al. Zinc finger transcription factor 191, directly binding to β-catenin promoter, promotes cell proliferation of hepatocellular carcinoma[J]. Hepatology,2012,55(6):1830-1839. doi: 10.1002/hep.25564 [29] LIU Y F, WU D, CHENG H H, et al. Wnt8B, transcriptionally regulated by ZNF191, promotes cell proliferation of hepatocellular carcinoma via Wnt signaling[J]. Cancer Sci,2021,112(2):629-640. doi: 10.1111/cas.14738 [30] WU D, LIU G, LIU Y, et al. Zinc finger protein 191 inhibits hepatocellular carcinoma metastasis through discs large 1-mediated yes-associated protein inactivation[J]. Hepatology (Baltimore, Md.),2016,64(4):1148-1162. doi: 10.1002/hep.28708 [31] SIEGEL R L, MILLER K D, JEMAL A. Cancer statistics, 2019[J]. CA A Cancer J Clin,2019,69(1):7-34. doi: 10.3322/caac.21551 [32] MIRANDA-FILHO A, LORTET-TIEULENT J, BRAY F, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study[J]. Lancet Diabetes Endocrinol,2021,9(4):225-234. doi: 10.1016/S2213-8587(21)00027-9 [33] PRETE A, BORGES DE SOUZA P, CENSI S, et al. Update on fundamental mechanisms of thyroid cancer[J]. Front Endocrinol (Lausanne),2020,11:102. doi: 10.3389/fendo.2020.00102 [34] XIONG J, JIANG P P, ZHONG L, et al. The novel tumor suppressor gene ZNF24 induces THCA cells senescence by regulating Wnt signaling pathway, resulting in inhibition of THCA tumorigenesis and invasion[J]. Front Oncol,2021,11:646511. doi: 10.3389/fonc.2021.646511 [35] YOUN H J, HAN W. A review of the epidemiology of breast cancer in Asia: focus on risk factors[J]. Asian Pac J Cancer Prev,2020,21(4):867-880. doi: 10.31557/APJCP.2020.21.4.867 [36] PHILLIPS C, JEFFREE R, KHASRAW M. Management of breast cancer brain metastases: a practical review[J]. Breast,2017,31:90-98. doi: 10.1016/j.breast.2016.10.006 [37] KIMBUNG S, LOMAN N, HEDENFALK I. Clinical and molecular complexity of breast cancer metastases[J]. Semin Cancer Biol,2015,35:85-95. doi: 10.1016/j.semcancer.2015.08.009 [38] EARLY BREAST CANCER TRIALISTS' COLLABORATIVE GROUP (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials[J]. Lancet,2012,379(9814):432-444. doi: 10.1016/S0140-6736(11)61625-5 [39] JIA D, HASSO S M, CHAN J, et al. Transcriptional repression of VEGF by ZNF24: mechanistic studies and vascular consequences in vivo[J]. Blood,2013,121(4):707-715. doi: 10.1182/blood-2012-05-433045 [40] HOU L M, CHEN M S, YANG H W, et al. miR-940 inhibited cell growth and migration in triple-negative breast cancer[J]. Med Sci Monit,2016,22:3666-3672. doi: 10.12659/MSM.897731 -




 下载:
下载: