-
20世纪60年代末期,美国科学家Barnett Rosenberg在研究电场对细菌生长影响的实验中,首次观察到铂化合物能抑制细胞生长的现象,从而揭开了铂类抗肿瘤药物发展的序幕。经过40多年的临床验证,顺铂、卡铂等铂类化合物在恶性肿瘤化疗中的作用得到充分确认。顺铂被发现具有抗癌活性,但其肾、胃肠道等毒性较为严重。在顺铂的结构基础上加以改造,第二代铂类抗肿瘤药物卡铂问世。卡铂降低了顺铂的毒性、但仍有较重的毒副作用,而且和顺铂交叉耐药,限制了其临床应用[1]。
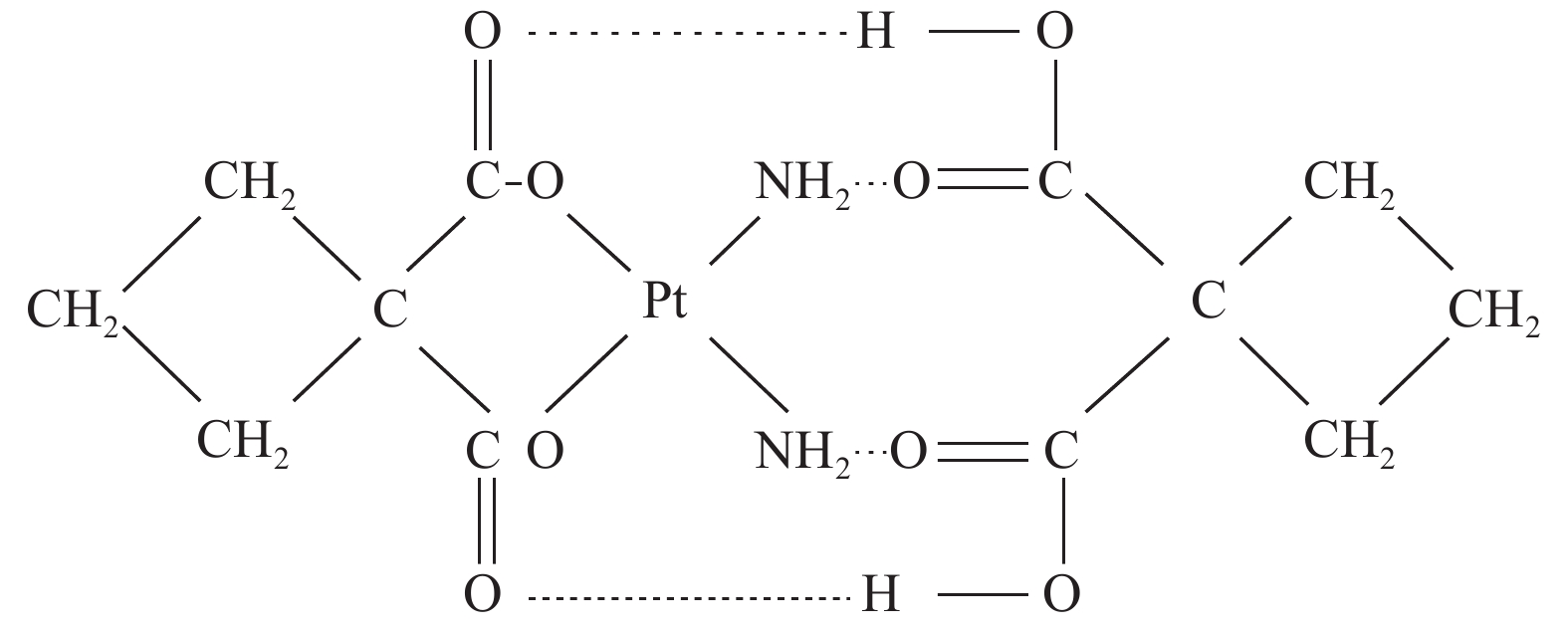
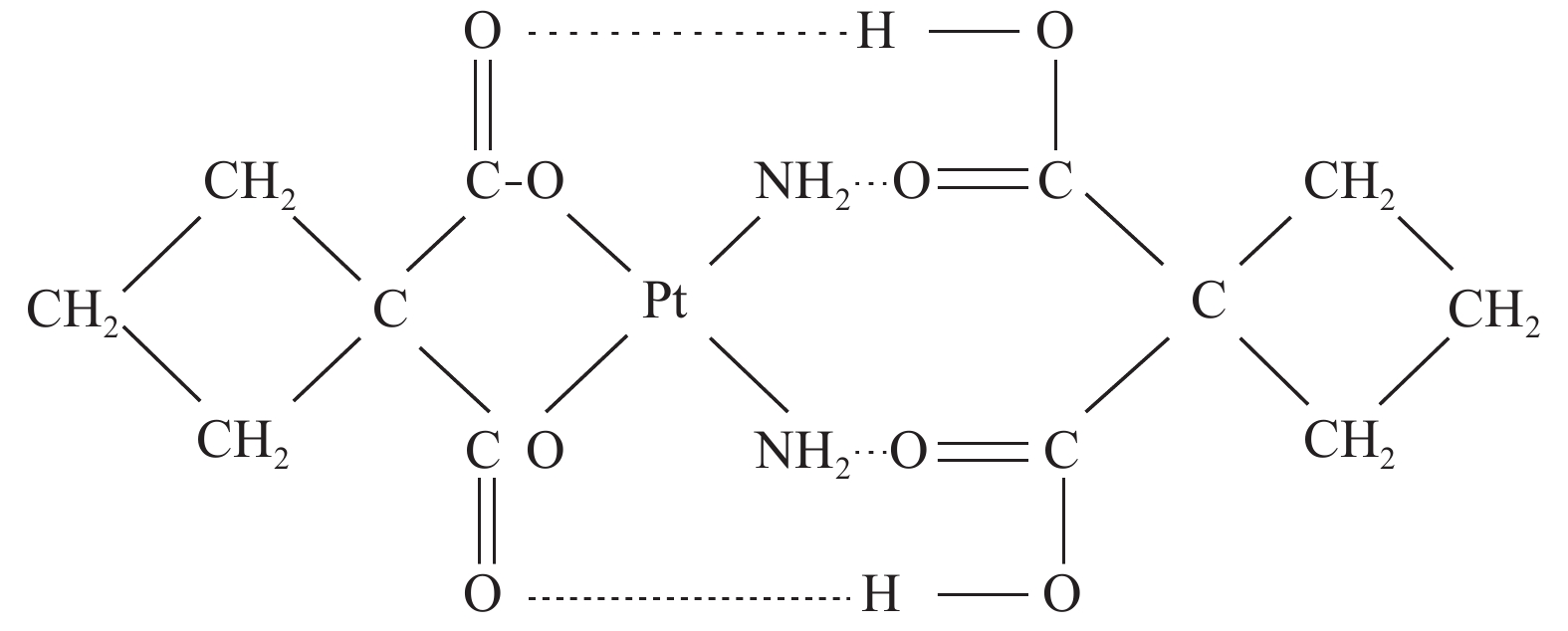
双环铂是我国自主研发的铂类化合物,是继顺铂、卡铂之后的新型铂类抗癌药物。双环铂化学名为顺-(1,1-环丁二羧酸)二氨合铂(Ⅱ),其分子结构(见图1[2])中除含环丁二羧酸基团外,同时以强氢键结合另外一个环丁二羧酸而形成铂络合物。
双环铂作为超分子笼状化合物,可在人体内导向性地与异常DNA的碱基络合,靶向准确杀死癌细胞,发挥抗癌治疗作用。体外细胞和动物实验发现双环铂对多种癌症细胞均有抑制激增的作用,对肝癌、胃癌、前列腺癌、卵巢癌、肺癌等具有较强的抗肿瘤活性,且毒副作用较同类铂络合物低[3-5]。鉴于该药临床应用时间短,相关参考文献较少,本文对我院收治使用双环铂治疗恶性肿瘤患者的疗效及副反应等进行了回顾性总结,为临床用药提供参考。
HTML
-
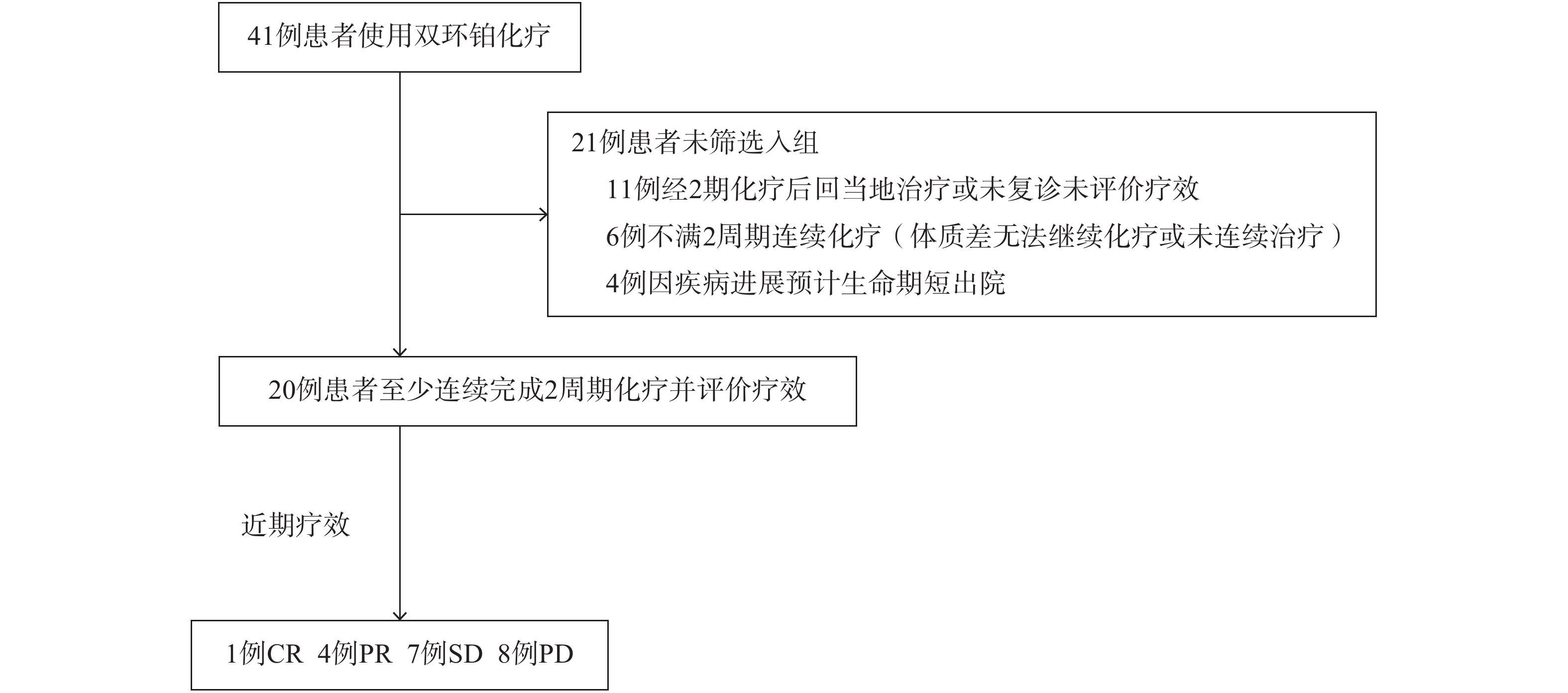
收集2017年1月至2018年8月我院收治使用双环铂治疗的肿瘤患者,41例患者中满足入组条件共20例。其中男性16例,女性4例,年龄18~85岁,中位年龄57.85岁。其中肺癌8例,食管癌1例,胰腺癌2例,膀胱癌1例,胸腺瘤1例,鼻咽癌1例,胃癌1例,淋巴瘤1例,前列腺癌1例,子宫内膜癌1例,脑肿瘤2例。入选标准:①入组患者经病理学或细胞学检查确诊,已经手术或不具备手术条件的肿瘤患者;②化疗前血常规、肝肾功能、心电图等基本正常,符合化疗标准;③KPS评分≥70分,预计生存期≥3个月;④至少以双环铂注射液为基础连续完成2个周期化疗,有明确客观评价指标,可评价近期疗效,筛选详情见图2,患者一般临床特征统计见表1。
项目 数量[n(%)] 项目 数量[n(%)] 年龄(岁) 57.85(18−85) 转移部位 <50 5(25) 内脏 9(45) ≥50−60 5(25) 肝脏 1(5) >60 10(50) 仅淋巴结节 4(20) 性别 多发脏器转移 6(30) 女 4(20) 骨转移 1(5) 男 16(80) 双环铂治疗周期 初/复诊 2期 2(10) 初诊 3(15) 3期-4期 11(55) 复诊 17(85) >5期 7(35) KPS评分 其他同期用药 ≤70 6(30) 化疗药 8(40) 70-80 0 PD-1 11(55) 90 14(70) 无 5(25) -
入组患者化疗2~4个周期后根据CT、超声检查确定可测量病灶的变化,按照WHO实体瘤的近期疗效RECIST1.0标准评定疗效。完全缓解(CR):所有目标病灶消失;部分缓解(PR):基线病灶长径总和缩小≥30%;稳定(SD):基线病灶长径总和缩小但未达PR;进展(PD):基线病灶长径总和≥20%或出现新病灶。疾病缓解率(RR)=CR+PR,疾病控制率(DCR)=CR+PR+SD,毒副反应按WHO抗癌药物制定的毒性评定标准分为0~Ⅳ度[6]。根据卡氏评分(Karnofsky performance status,KPS)对患者治疗前和化疗2周期后的生活质量进行评估,总分为0~100分,得分越高表示生活质量越好。KPS评分较治疗前增加≥10分为改善,减少≥10分为下降,增加或减少在10分以内为稳定。t检验用于检验用药前后疗效分析结果,当P<0.05时,表明用药效果显著。
1.1. 一般对象
1.2. 评价标准
-
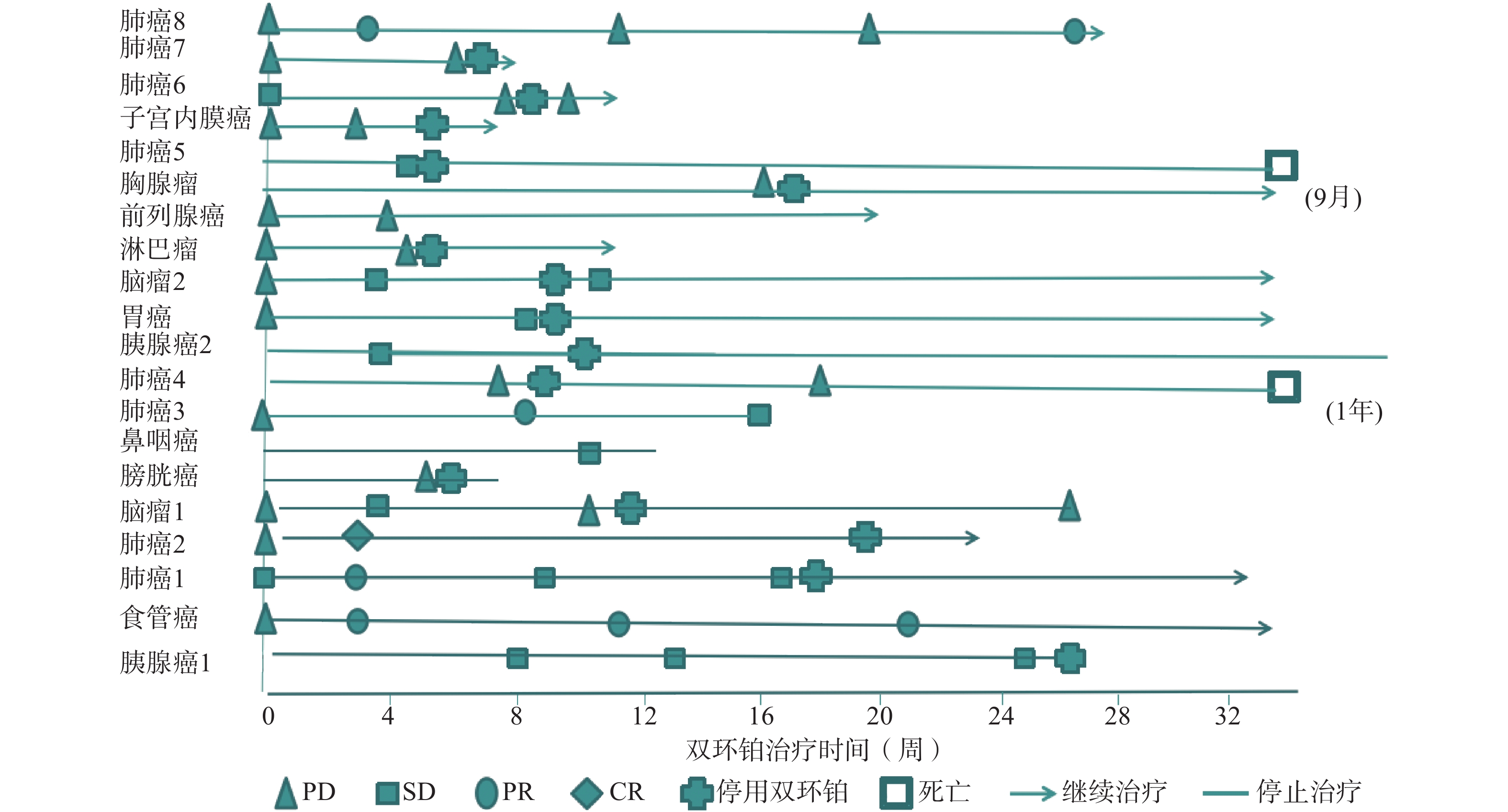
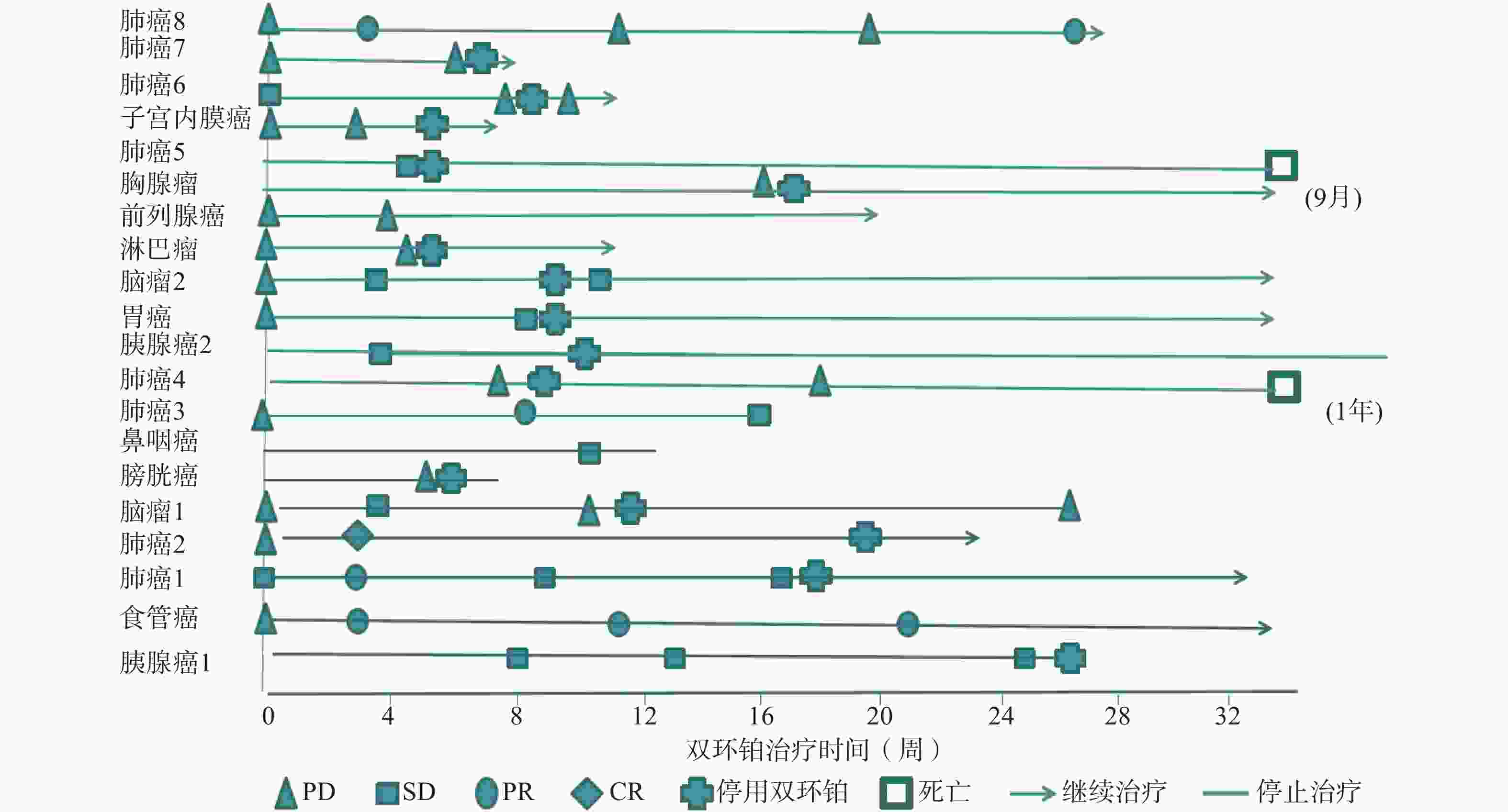
全组患者均可评价近期疗效,20例患者中有11例患者在处于疾病进展(PD)期开始使用双环铂单药或联合用药治疗。入组患者中近期疗效评价完全缓解(CR) 1例(5%),为肺癌;部分缓解(PR)4例(20%),其中肺癌3例,食管癌1例;稳定(SD)7例(35%),其中胰腺癌2例、鼻咽癌1例、胃癌1例、肺癌1例、脑肿瘤2例;进展(PD)8例(40%),其中膀胱癌1例、肺癌3例、淋巴瘤1例、前列腺癌1例、胸腺瘤1例、子宫内膜癌1例。总有效率RR(CR+PR)为25%,疾病控制率DCR(CR+PR+SD)为60%,其中一例肺腺癌患者在经一线(卡铂+紫杉醇)、二线(依托泊苷+顺铂)治疗获益6个月后处于疾病进展(PD),肿瘤标记物明显升高的情况下换用双环铂+伊立替康化疗3周期后评价PR。不同肿瘤有效率、控制率见表2。患者化疗联合用药有紫杉醇(白蛋白结合型)、氟尿嘧啶、尼妥珠单抗、贝伐珠单抗、帕博利珠单抗等。有4例同时联合其他化疗药和帕博利珠单抗,其中食管癌1例,评价PR;肺癌1例,评价PR;胃癌1例,评价SD;子宫内膜癌1例,评价PD。联合用药的近期疗效评估见表3,患者经双环铂治疗周期的疗效变化见图3。
肿瘤类型 例数 完全缓解 部分缓解 稳定 进展 缓解率(%) 控制率(%) 肺癌 8 1 3 1 3 50 62.5 子宫内膜癌 1 1 0 0 胰腺癌 2 2 0 100 脑瘤 2 2 0 100 鼻咽癌 1 1 0 100 胸腺瘤 1 1 0 0 淋巴瘤 1 1 0 0 膀胱癌 1 1 0 0 前列腺癌 1 1 0 0 胃癌 1 1 0 100 食管癌 1 1 100 100 合计 20 1 4 7 8 25 60 疗效 用药例数(占比) 联合化疗药 联合单抗PD-1 单用 完全缓解(CR) 1 部分缓解(PR) 4 2 稳定(SD) 3 3 2 进展(PD) 1 5 3 合计 8(40%) 11(55%) 5(25%) -
根据KPS评分标准,治疗后KPS评分为(88±10.95),高于治疗前的(80.5±10.33),差异有统计学意义(P<0.05)。
-
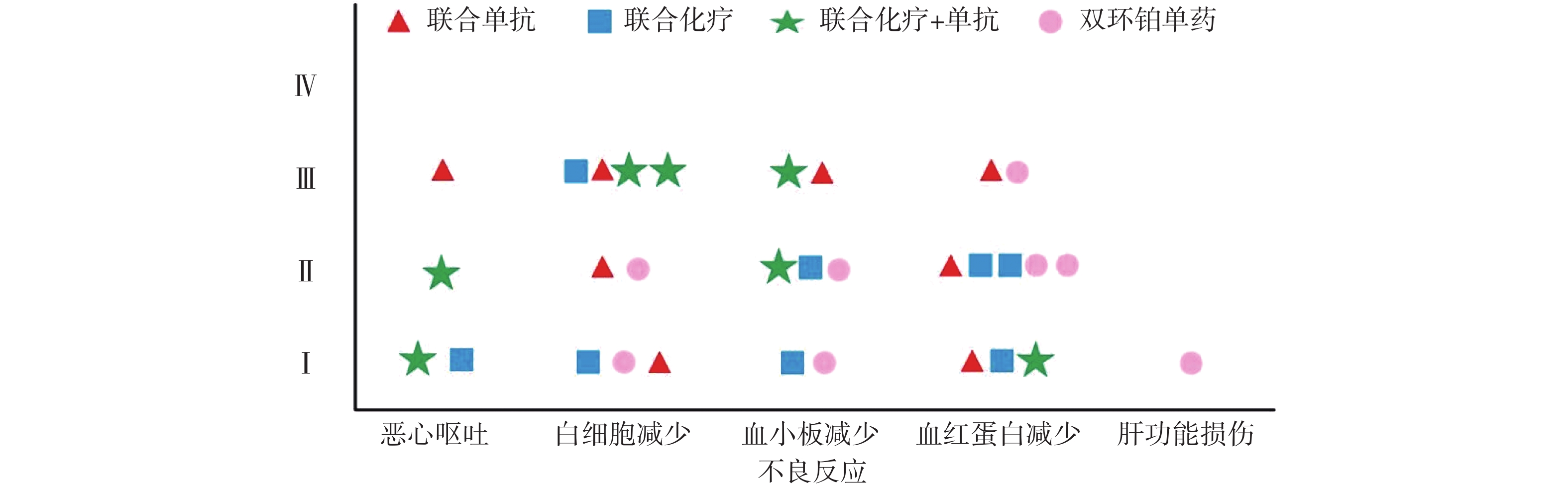
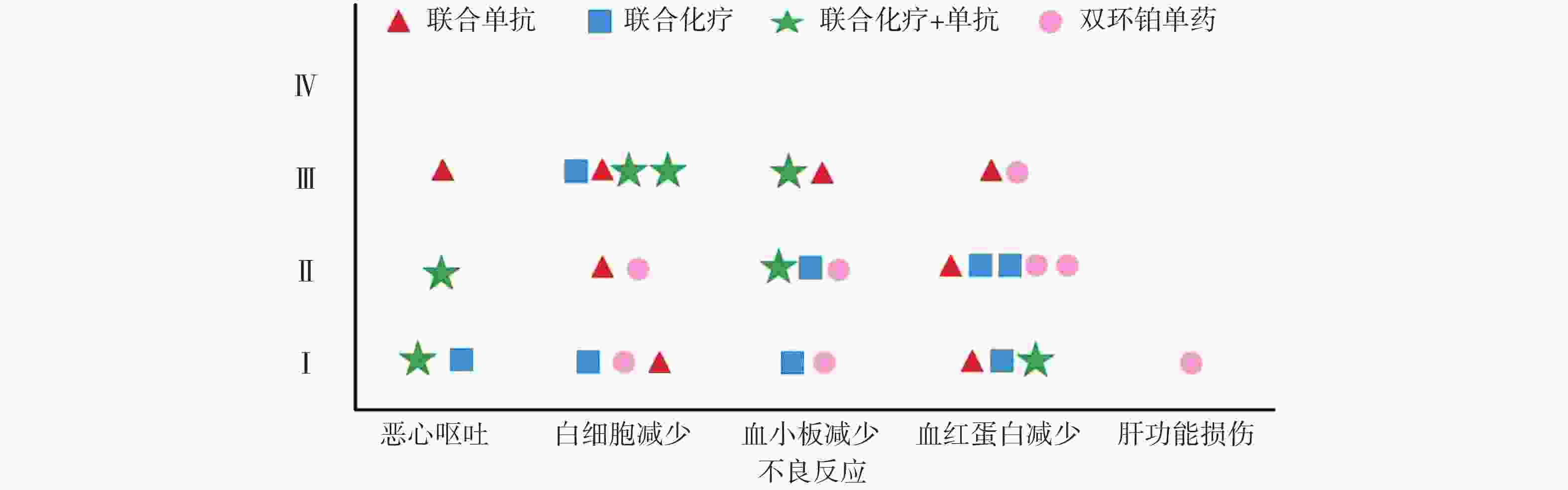
全组共完成80个周期化疗,毒副作用骨髓抑制和消化道反应最为常见,多为Ⅰ~Ⅱ度,无Ⅳ度。20例患者中白细胞减少发生率为45%,血小板和血红蛋白减少率分别为35%和50%。白细胞Ⅰ、Ⅱ度减少5例占25%,血红蛋白Ⅰ、Ⅱ度减少有8例占40%。消化道反应主要表现是恶心呕吐,其中Ⅰ度2例占10%,Ⅱ度、Ⅲ度各1例占比5%,Ⅰ度肝功能损伤1例占5%,详见表4和图4。
类型 不良反应例数 发生率(%) 无 Ⅰ Ⅱ Ⅲ Ⅳ 恶心呕吐 16 2 1 1 0 20 白细胞减少 11 3 2 4 0 45 血小板减少 13 2 3 2 0 35 血红蛋白减少 10 3 5 2 0 50 肝功能损伤 19 1 0 0 0 5
2.1. 近期疗效
2.2. 治疗前后生活质量比较
2.3. 毒副作用
-
据肿瘤流行病学资料显示,肺癌是对人类生命和健康威胁最大的恶性肿瘤之一,筛选入组的20例患者中有8例是肺癌患者。非小细胞肺癌是肺癌最常见的组织学类型,约占肺癌总数的80%,且多数患者就诊时已近晚期,局部病变发生转移失去手术机会。对于体能状态好,不可手术切除的肺癌患者推荐以铂类为基础的联合化疗是肺癌治疗的一线方案。目前,卡铂联合紫杉醇治疗晚期非小细胞肺癌方案较为成熟,Chen等[7]报道该方案治疗晚期非小细胞肺癌有效率为40%。一项比较双环铂联合紫杉醇方案与卡铂联合紫杉醇方案治疗非小细胞肺癌的临床研究显示:两组的有效率和中位疾病进展时间没有显著差异,但试验组双环铂的3年生存率显著优于对照组卡铂[8]。
晚期肿瘤主要的治疗手段是化疗,选择合理的化疗方案可提高患者生活质量,延长生存期。本文选用双环铂化疗方案治疗的20例患者中,单药治疗占比25%,联用其他化疗药(紫杉醇、氟尿嘧啶等)占比40%,联用单抗(帕博利珠、尼妥珠、贝伐珠单抗等)占比55%,其中20%患者同时联合化疗药和帕博利珠单抗治疗。帕博利珠单抗是近几年出现的一种新型抗肿瘤免疫药物,国际多项临床研究表明该类药物在肺癌、恶性黑色素瘤、头颈部肿瘤中显示出较好的疗效。本研究入组患者大于3线治疗共13例(65%),且多为肿瘤晚期或经手术放化疗后复发伴脏器、淋巴等多发转移,治疗后患者生活质量提高,疗效总有效率为25%,疾病控制率达60%。从图3可见20例患者中有11例患者在评估疾病进展(PD)后选用双环铂单药或联合用药治疗后评价近期疗效,其中CR 1例、PR 3例、SD 3例,有效率和疾病控制率分别是36%和64%,这说明双环铂在抑制肿瘤生长方面具有一定的优势,是有前途的抗癌药物。
本研究中消化道反应和骨髓抑制是化疗副反应中最为常见和严重的,这与双环铂临床前动物实验研究发现的主要毒性是血液系统毒性和消化系统毒性相吻合[9]。从图4可见本组研究中骨髓抑制的不良反应主要集中在白细胞减少和血红蛋白减少,其次是血小板减少,程度为Ⅰ~Ⅲ级。Ⅲ度骨髓抑制发生率较高,多在化疗3周后出现,双环铂联合单抗治疗者发生的概率较大。全组无Ⅳ度骨髓抑制和胃肠道反应,无过敏反应、无严重肾功能损伤。消化道反应主要表现在恶心呕吐,这也是很多肿瘤患者恐惧化疗的重要原因之一。本组病例化疗前后给予患者盐酸帕洛司琼注射液、盐酸昂丹司琼注射液、注射用托烷司琼等止吐用药,因此发生恶心呕吐的不良反应率较低,且绝大部分患者可以耐受,不影响后续治疗。其他辅助用药包括细胞保护,预防过敏,预防神经毒性,保肝、营养支持及对症处理,所有症状对症治疗后好转,多数患者经对症治疗后均可耐受。
综上所述,双环铂以其高效、低毒的特性可用于很多晚期肿瘤的治疗,特别是为多次化疗后不能耐受或产生多药耐药的癌症患者提供了新的用药选择。但本组病例数不多,单药治疗病例数较少,双环铂治疗的远期疗效及不良反应有待继续观察,值得开展大样本临床研究以进一步评价其疗效和毒性。








 DownLoad:
DownLoad: