-
冷损伤在冬季户外作业人员中常见,长时间冷暴露会造成不可逆性损伤甚至危及生命。目前,对冷损伤的研究主要侧重在肢端损伤的对症治疗,尚缺乏针对体温和机体功能下降的干预策略。冷暴露过程中,核心体温下降是导致冷损伤最直接的因素。核心体温降至35 ℃时,则损伤开始[1]。核心温度为33~35 ℃则发生轻度低温,降至28~32 ℃为中度低温、17~27 ℃为深度低温。核心体温下降将引起重要器官、组织发生系列生理和病理变化,造成不同程度的损伤[2]。然而,目前维持核心体温的主要方式为穿戴保暖装备[3],有效维持核心体温的抗寒药物研究较少。因此,通过药物增加机体的产热作用,可能是预防冻伤的一种有效方法。
外界温度降低将导致基础产热无法维持体温恒定,继而发生颤抖性产热(shivering thermogenesis,ST)和非颤抖性产热(non-shivering thermogenesis,NST)[4]。与ST相比,NST可以持续产热且不会产生明显疲劳等不适感。棕色脂肪组织(brown adipose tissue,BAT)作为NST的最重要来源,在冷暴露中发挥着重要的调节产热能力[5]。当NST激活时,BAT线粒体内膜上的UCP1表达水平增多,线粒体中电子传递链断开ATP生成减少,使化学能转化为热能[6],从而增加机体产热、维持核心体温[7]。
米格列醇(miglitol)为第二代半合成α-糖苷酶抑制剂[8],结构与葡萄糖相似,通过延长餐后小肠中碳水化合物的吸收发挥降糖作用,为临床常用的口服抗糖尿病药物[9]。有证据表明,米格列醇有减脂作用[10, 11],这可能与其改变肠道中短链脂肪酸的生成有关[12]。近年研究表明,米格列醇具有独立于延缓碳水化合物吸收的减重机制[13]。米格列醇抑制脂质积累的机制可能是通过增加脂肪组织产热实现[14]。因此米格列醇对BAT的能量消耗水平、产热激活以及体温调节作用机制值得进一步探讨。
本研究以诱导分化成熟的棕色脂肪细胞和冷暴露小鼠为研究对象,探究米格列醇对棕色脂肪细胞增殖和脂质消耗的影响,考察冷暴露小鼠体温以及产热通路关键蛋白的变化,为深入探究米格列醇产热作用机制提供理论依据,为冷损伤提供干预策略。
-
CO2 培养箱(力康生物医疗科技控股有限公司);电热恒温水浴锅(DK-98-11,天津市泰斯特仪器有限公司);水平层流洁净工作台(上海上净净化设备有限公司);荧光倒置显微镜(日本IX71,Olympus公司);酶标测试仪(ELX-800,上海伯乐生命医学产品有限公司);动物肛温测定仪(ZS-DTY,北京众实迪创科技发展有限公司);红外热成像仪(E5,美国菲力尔公司);恒温实验动物冰箱(RXZ-0250,海尔公司);电泳仪、转膜仪(北京六一仪器厂);电泳凝胶成像分析系统(美国Bio-Rad公司);
细胞培养及活性测定相关试剂:Ⅱ型胶原酶(北京索莱宝科技有限公司);牛血清白蛋白(北京Biosharp公司);HEPES(北京索莱宝科技有限公司);红细胞裂解液(上海碧云天生物技术有限公司);DMEM 培养基(美国Hyclone公司);FBS(美国Clark公司);胰酶、胰岛素、3-异丁基-1-甲基次黄嘌呤(IBMX,纯度≥98%)、地塞米松(dexamethasone, Dex,纯度≥98%)、EDTA(美国Sigma公司),油红O染色试剂盒(沈阳万类生物科技有限公司)。
蛋白印迹(Western blot)相关试剂:脂肪组织蛋白提取试剂盒(德国Invent公司),BCA蛋白浓度测定试剂盒(上海碧云天生物技术有限公司);Western blot相关试剂和抗体:甘氨酸、Tris-HCl缓冲液(pH 8.8)、Tris-HCl缓冲液(pH 6.8)、20×TBS、Tween 20(北京索莱宝科技有限公司);十二烷基硫酸钠(SDS)、过硫酸铵(AP)(美国Sigma公司);N,N,N,N-四甲基乙二胺(TEMED)(美国Amresco 公司);30%丙烯酰胺(北京Biosharp公司);UCP1抗体(英国Abcam公司)、PGC1α抗体(沈阳万类生物科技有限公司);ECL化学发光底物(美国Bio-Rad公司)。
米格列醇(纯度≥98%,B25416,上海源叶生物科技有限公司)。
-
新生SD大鼠(24 h内)10只,用于原代前棕色脂肪细胞的提取和培养。取健康昆明小鼠(18~22 g),随机分组,每组10只,分成空白对照组、冷暴露对照组、米格列醇组(40 mg/kg)、阳性对照组-全反式维甲酸组(ATRA,40 mg/kg),给药7 d。米格列醇和ATRA的给药剂量,结合临床常用最大给药剂量并参考其脂肪酸氧化及代谢的相关文献[15, 16],并经过预实验确定。
-
采用参考文献[17]的方法,从24 h新生SD(Sprague-Dawley)大鼠BAT中分离原代前棕色脂肪细胞。新生SD大鼠置75%乙醇中浸泡5 min,取出BAT,置预冷的PBS中剪碎置胶原酶Ⅱ(1.5 mg/ml)中,37 ℃消化30 min。消化后的组织匀浆经300目尼龙筛网过滤并离心(800 r/min×5 min),沉淀用红细胞裂解液重悬后再次洗涤和离心。分离得到前脂肪细胞接种于DMEM(含10%胎牛血清)培养基中,在37 ℃、5% CO2条件下培养。细胞生长融合至80%~90%,更换为诱导分化液Ⅰ(含1 mmol/L Dex、0.5 mmol/L IBMX和1.67 mmol/L Insulin的DMEM培养液)培养48 h,再更换为诱导分化液Ⅱ(含1.67 mmol/L Insulin的DMEM培养液)培养48 h,于DMEM完全培养基中继续培养至细胞分化成熟、布满脂滴。
-
采用参考文献[18]的方法,通过MTT实验评估米格列醇对棕色脂肪细胞活力的影响。原代前棕色脂肪细胞按照1×10 4个/孔接种于96孔板中。培养24 h后,分别向样品孔中加入米格列醇或ATRA,孵育24、48、72 h。然后加入MTT溶液(终浓度为0.5 mg/ml)继续孵育4 h。结束后,弃去MTT溶液,加入200 μl DMSO溶解甲臜,在490 nm下测定各孔的吸光度。
-
采用参考文献[19]的方法,原代前棕色脂肪细胞接种于12孔板,分化成熟后,分别加入米格列醇及阳性药ATRA。48 h后,弃去培养液,细胞经10%中性甲醛溶液固定1 h后,加入油红O染色液,染色30 min。弃去染色液,双蒸水洗涤4次,拍照。各孔加入1 ml异丙醇萃取染色液,振荡10 min,在570 nm下测定各孔的吸光度A。脂滴消耗率=1−A样品/A对照。
-
为了减少粪便对测定的干扰,小鼠在核心体温测定前12 h禁食。4 ℃和−20 ℃冷暴露1 h后,立即用红外热成像系统拍摄并测量体表温度,并用FLIR tools软件进行体表平均温度的调取和分析。在冷环境下测定不同时间的小鼠核心体温并记录。采用肛温仪测定小鼠的肛温作为核心体温,肛温仪的探头涂抹适量凡士林,插入直肠约1.5 cm,读数稳定后快速操作并记录数据。
-
各组小鼠于给药7 d后,于−20 ℃恒温箱中冷暴露1 h取出至室温。3 h后测定小鼠足趾肿胀程度。
-
采用脂肪组织/细胞蛋白提取试剂盒提取总蛋白,通过酶标仪进行蛋白定量。配置分离胶和浓缩胶,经过上样、电泳、转膜、封闭、抗体孵育和曝光成像,对蛋白表达水平进行分析。
-
所有数据均以平均值±标准误(mean±SEM)表示,使用SPSS 17.0进行统计分析,采用GraphPad Prism 7.0(San Diego,USA)软件进行绘图。统计分析先对数据进行正态分布检验,组间比较采用单因素方差分析(One-way ANOVA),方差齐性采用Tukey检验,方差不齐则采用Dunnett’s T3检验。以P<0.05为具有统计学意义。
-
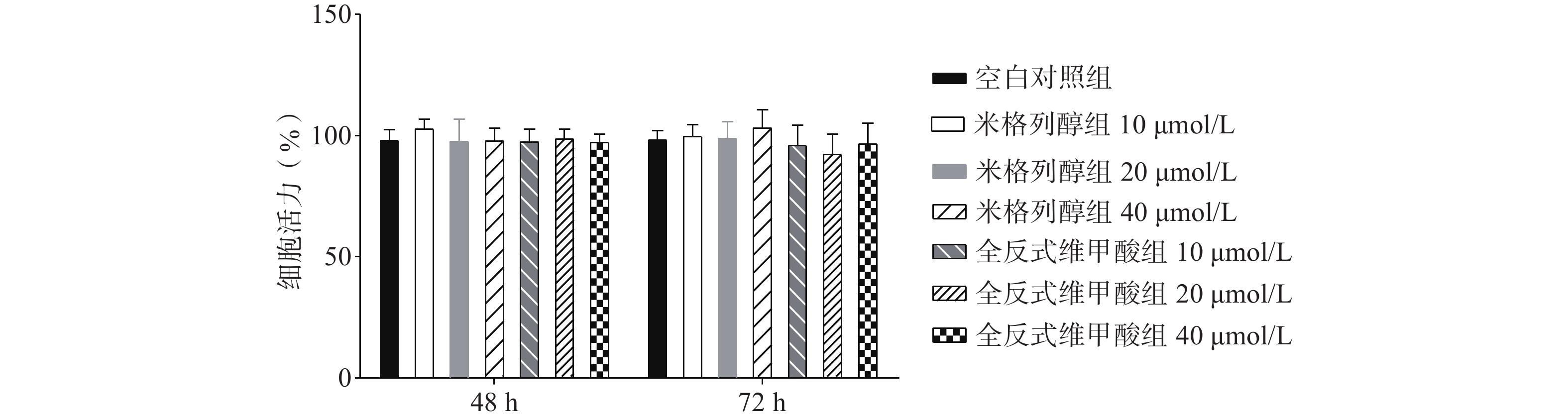
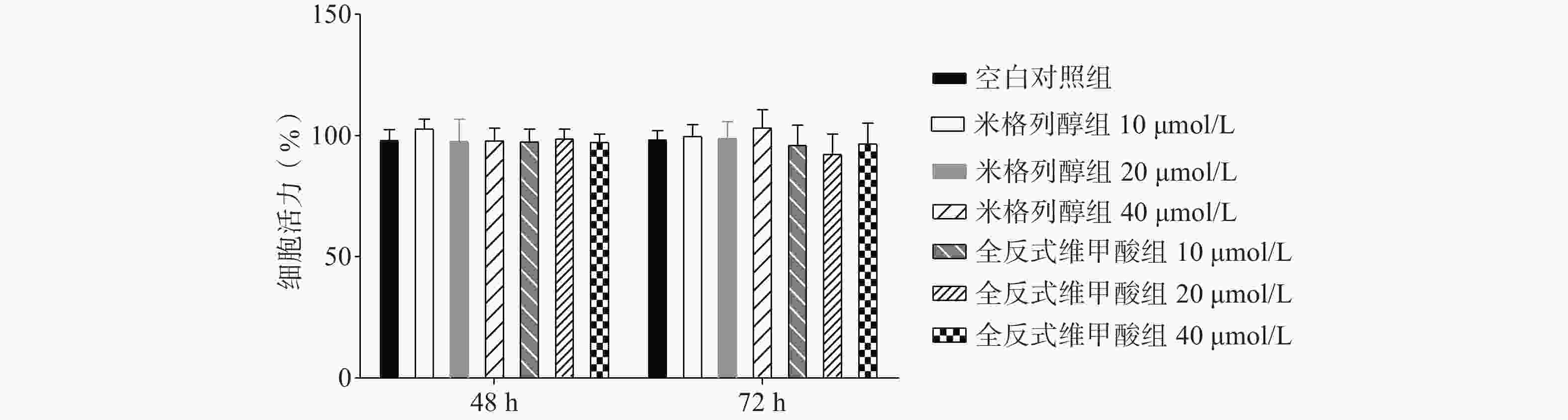
如图1所示,与空白对照组比较,根据参考文献[15, 20-22]中的给药浓度,本研究使用浓度分别为10、20、40 μmol/L的两种化合物处理原代棕色脂肪细胞,给药48 h、72 h后对细胞活力均无显著影响。说明选用40 μmol/L米格列醇和40 μmol/L阳性药全反式维甲酸并未影响脂肪细胞的增殖。
-
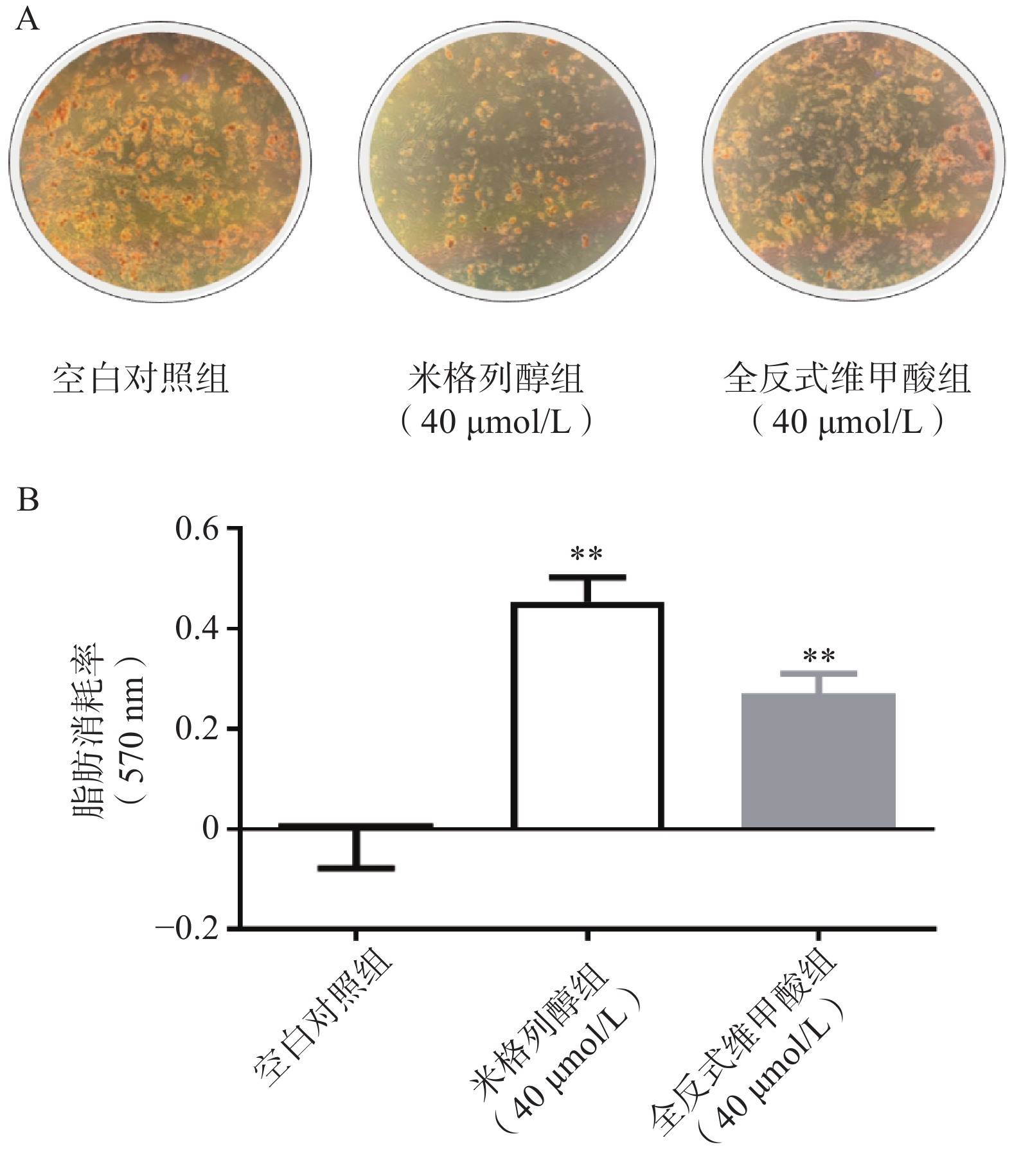
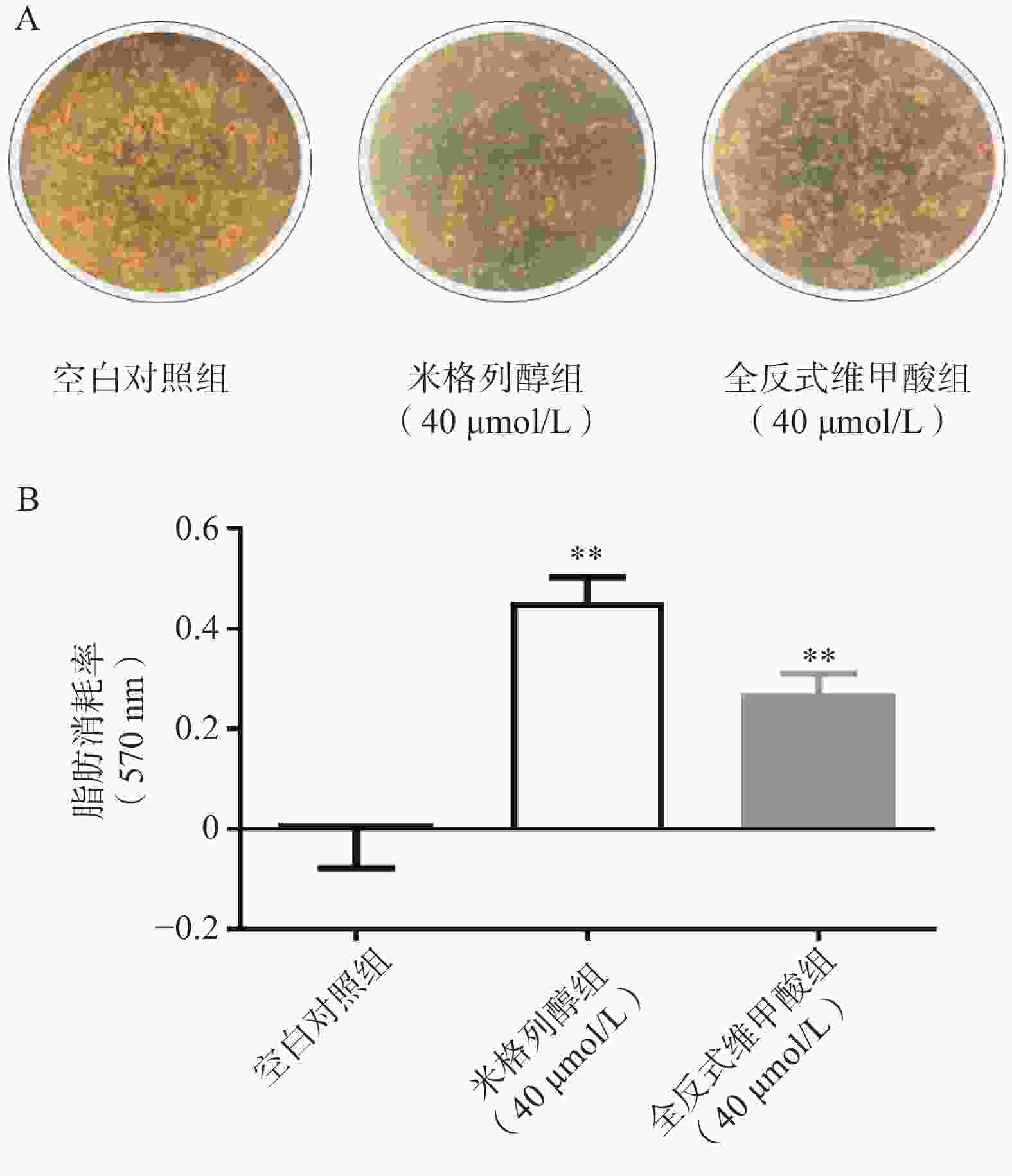
通过油红O染色的结果可知(图2),米格列醇显著增加了棕色脂肪细胞内的脂滴消耗水平,说明米格列醇提高了脂肪细胞内脂质的利用,消耗了产热底物。
-
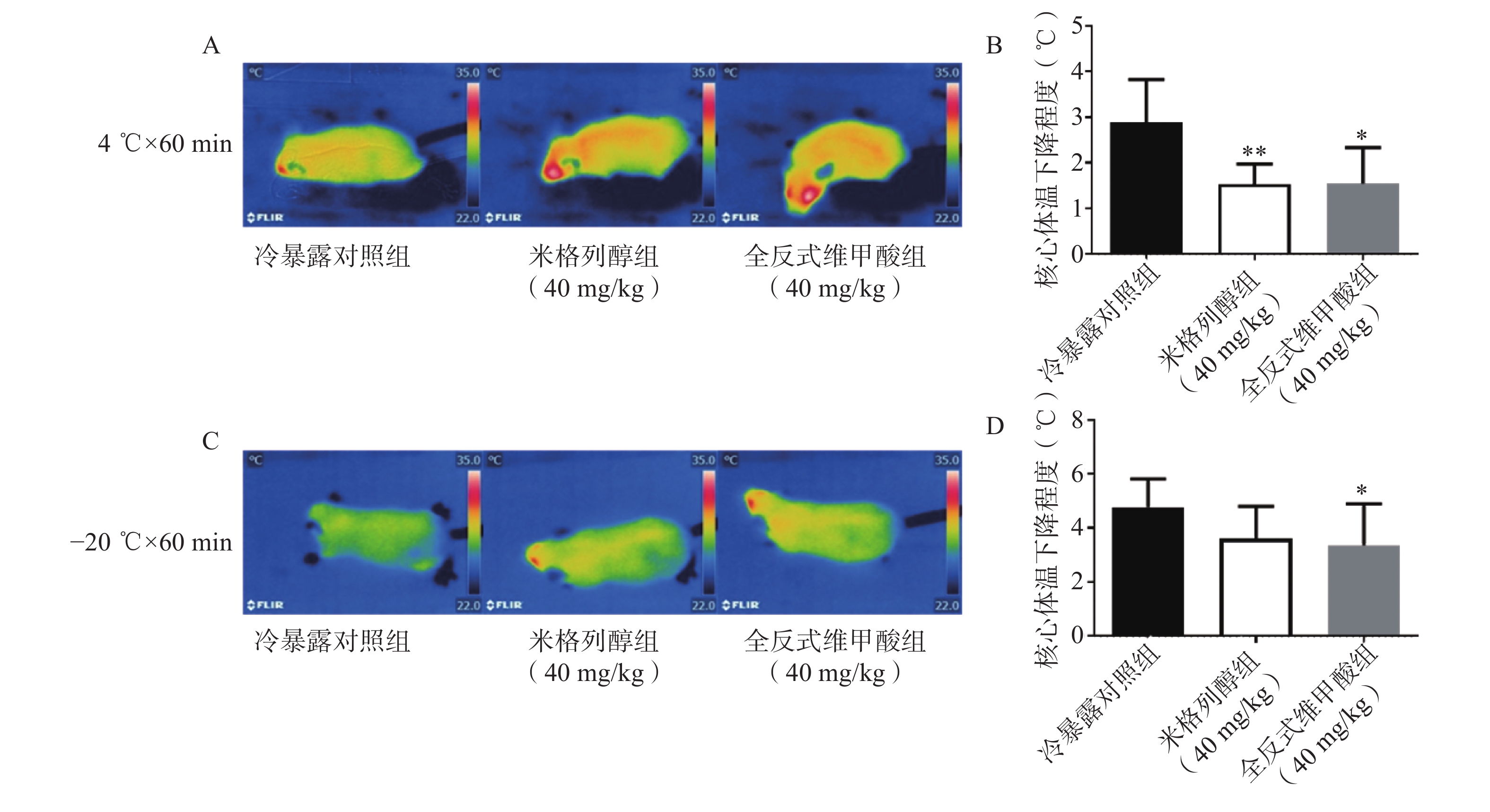
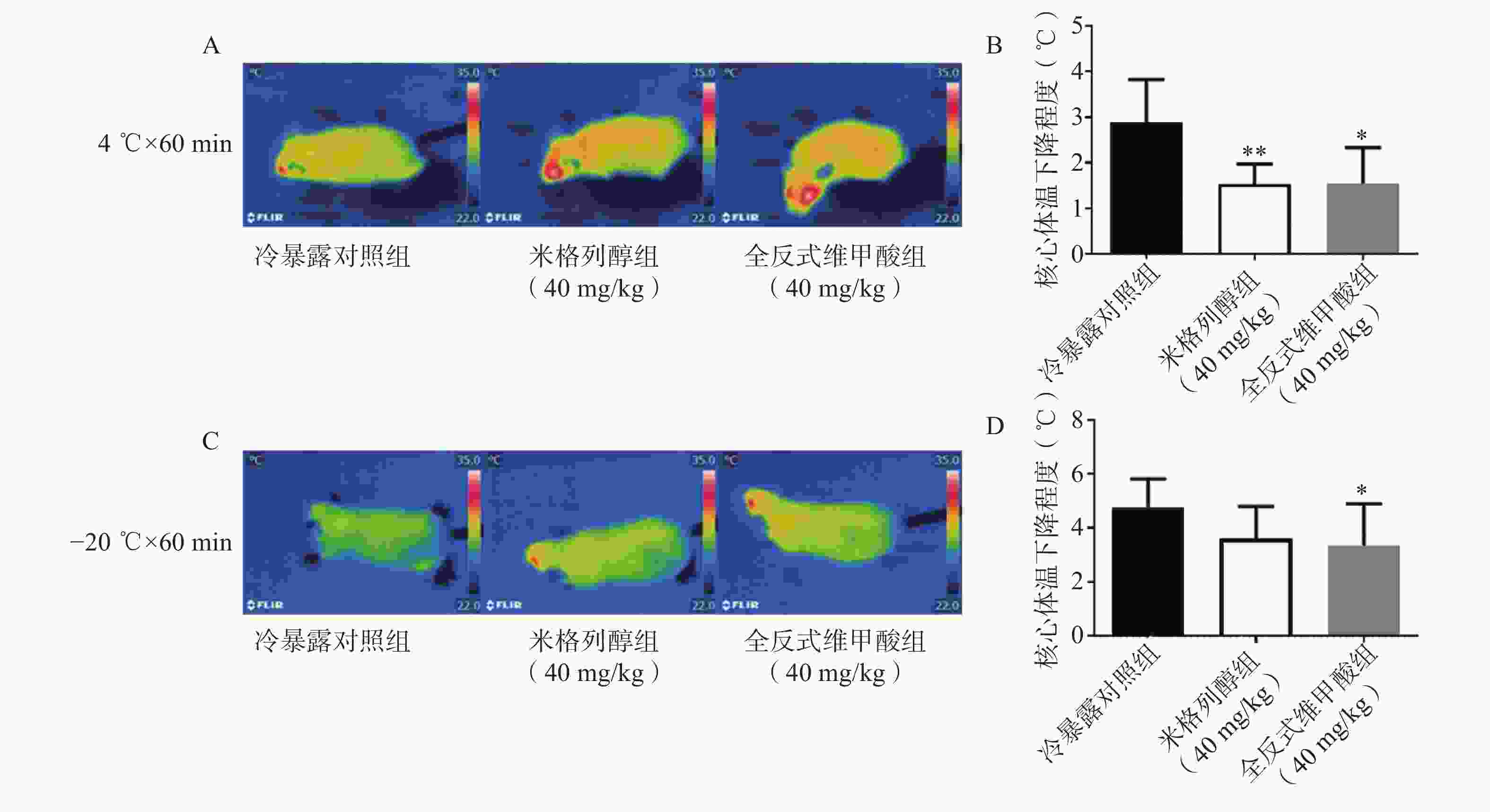
由红外热成像结果可知,给药7 d后的米格列醇显著提高了4 ℃冷暴露后小鼠的体表温度,也上调了核心温度。但对−20 ℃冷暴露后的体温并未有显著影响,见图3。
-
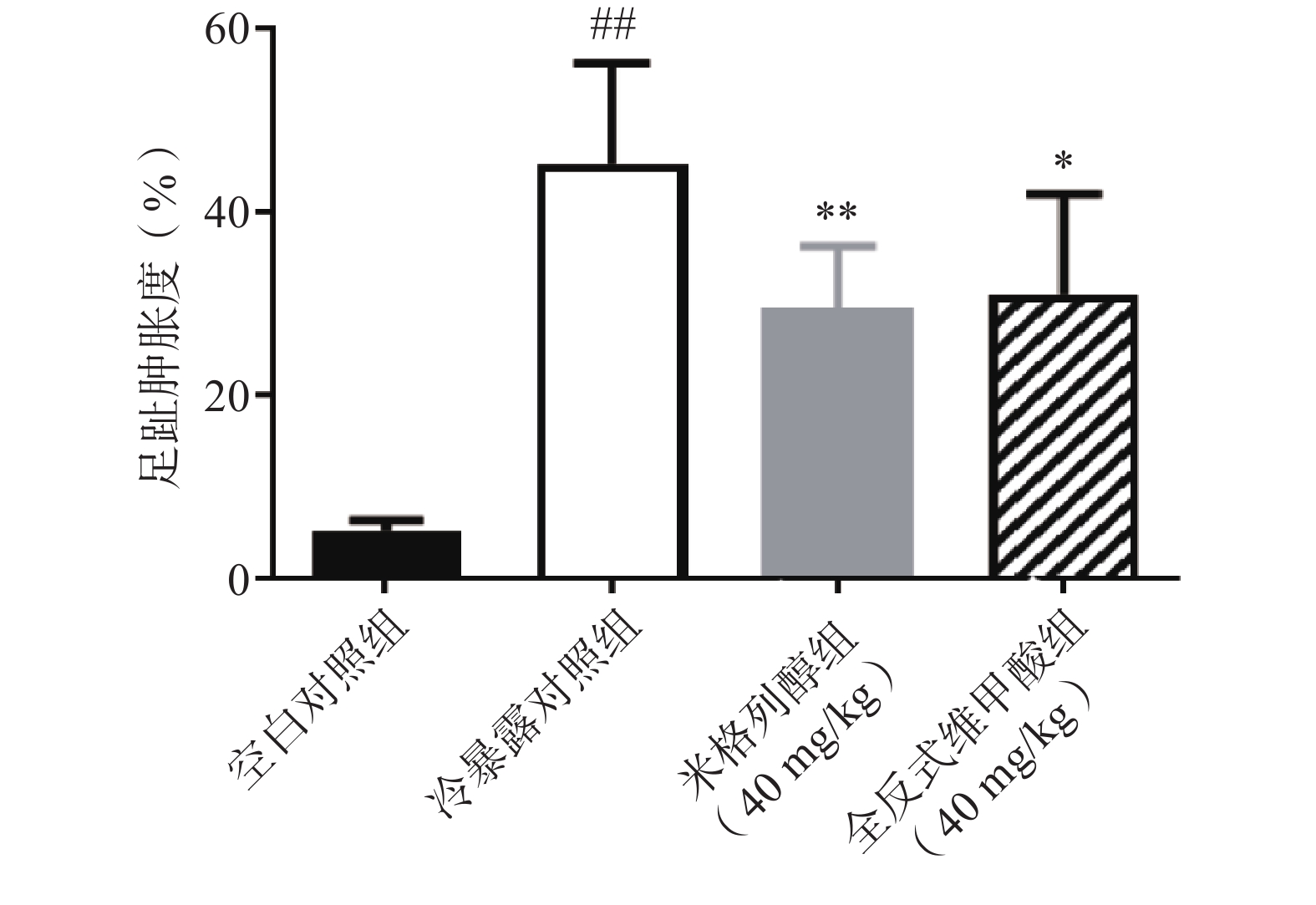
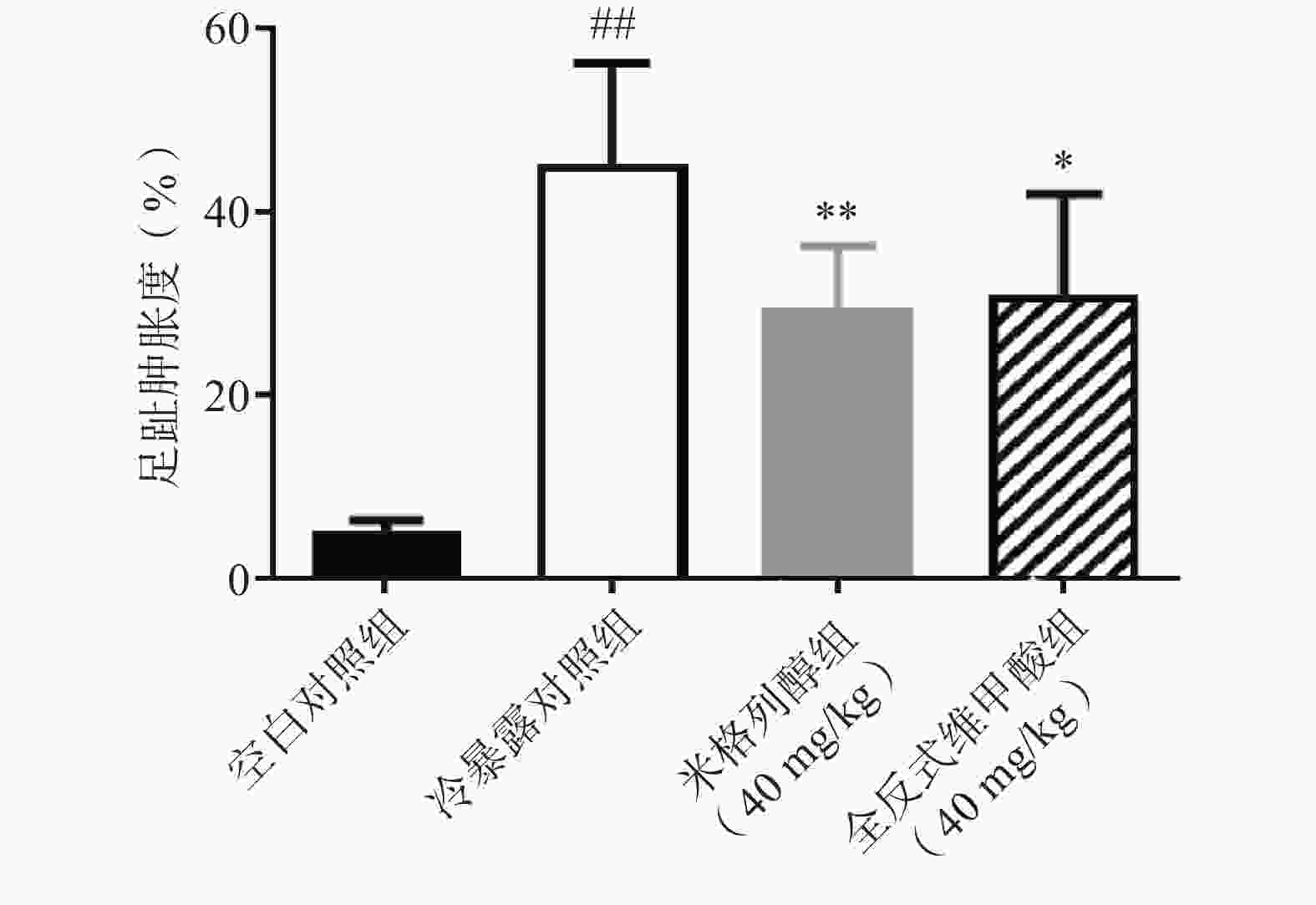
足趾肿胀程度可以反映小鼠寒冷损伤的程度,如图4所示,冷暴露对照组较空白对照组显著增加了小鼠的足趾肿胀程度,而预防给予米格列醇7 d可显著改善足趾肿胀程度,其改善作用较UCP1激动剂全反式维甲酸更明显。
-
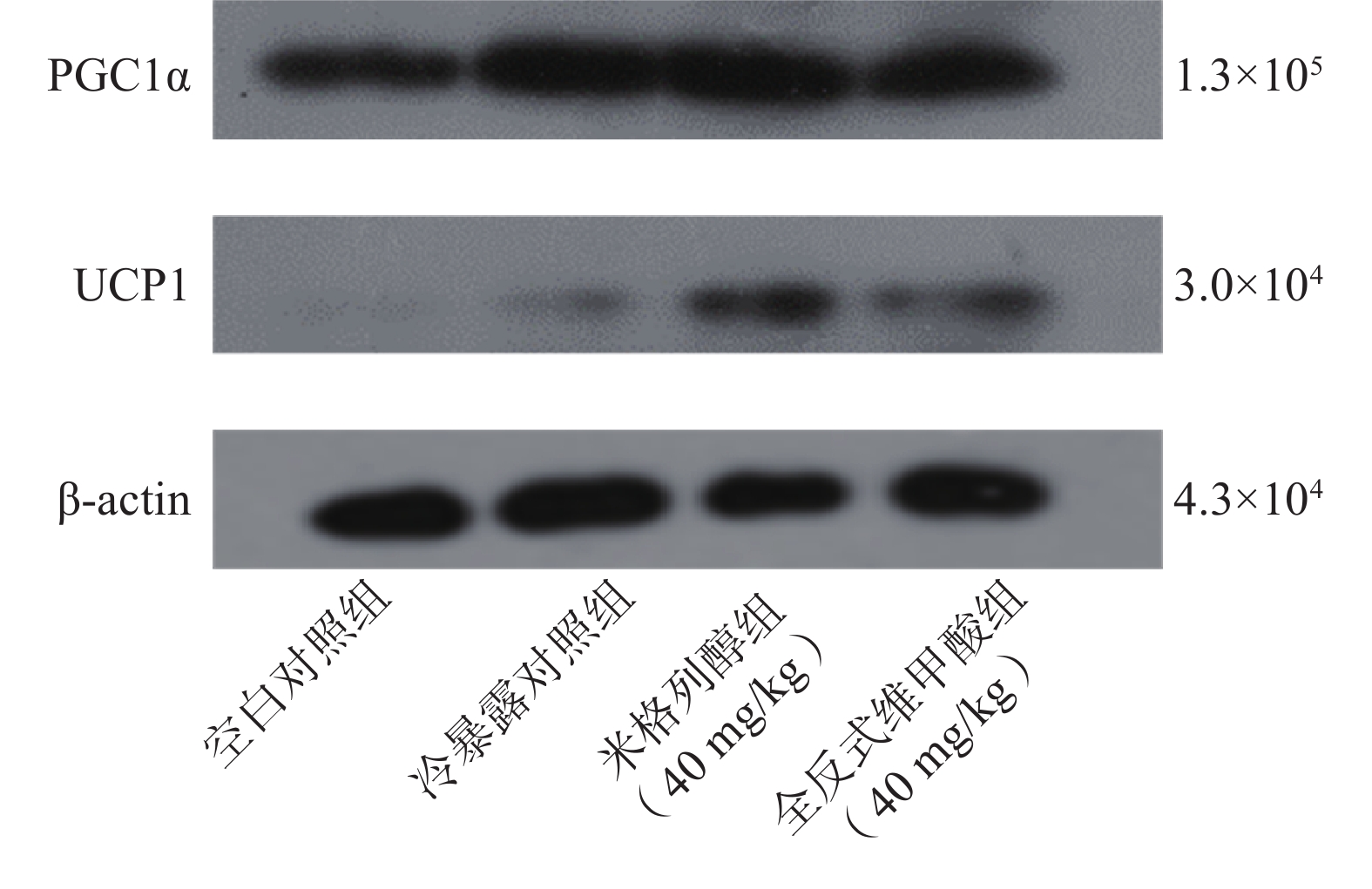
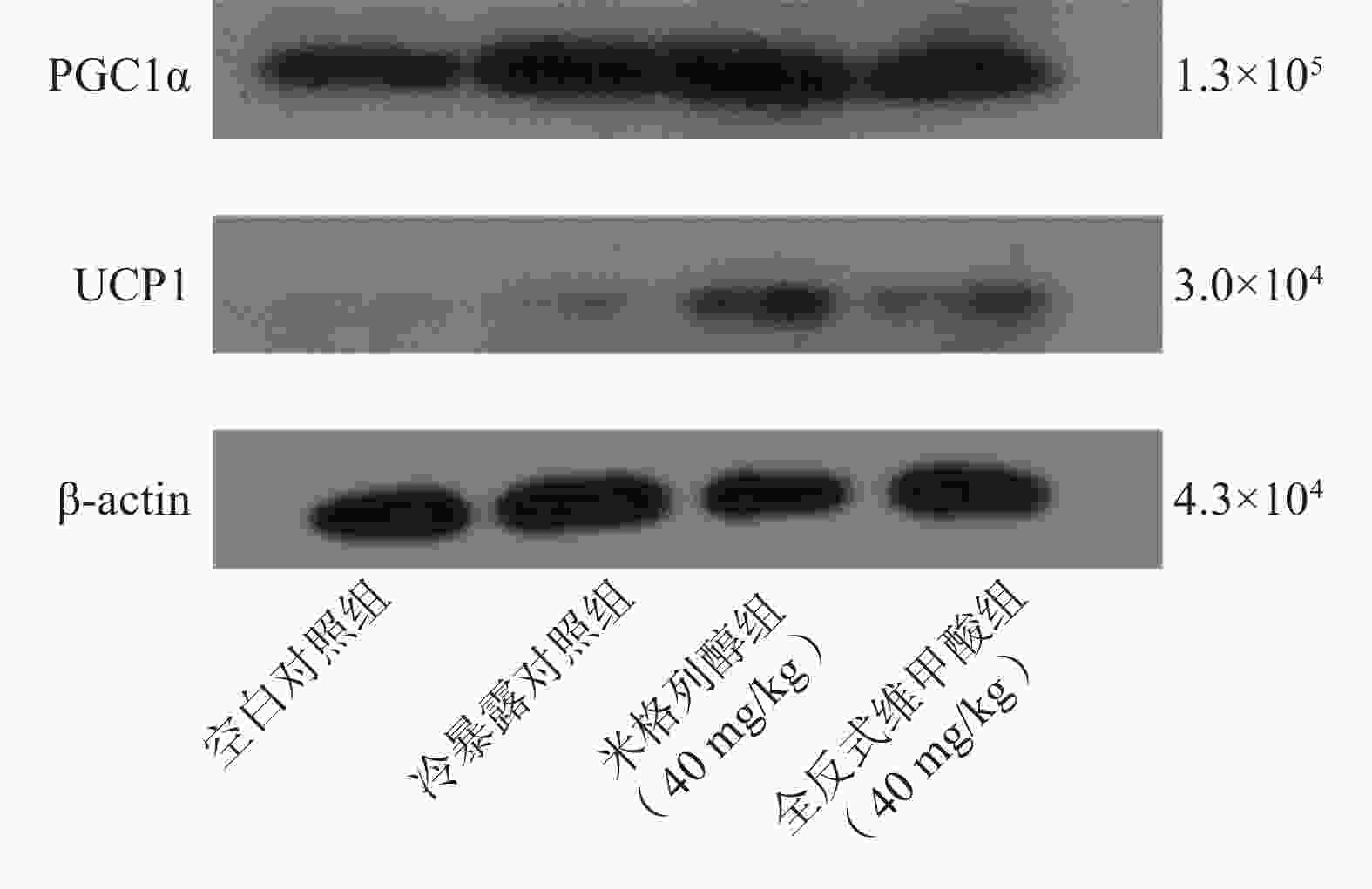
由蛋白印迹结果可知,与冷暴露对照组相比,米格列醇给药后可显著增加棕色脂肪组织中UCP1表达水平,且PGC1α的水平也有上调(图5),但对UCP1的表达水平调控作用较PGC1α更显著。
-
本研究通过体内外实验证实米格列醇可以通过激活UCP1的产热活性、增加脂肪组织的脂质消耗,从而进一步增加小鼠冷暴露后的体温、改善冷暴露引起的足趾肿胀程度。
米格列醇作为α-葡萄糖苷酶抑制剂是通过延缓肠道碳水化合物吸收而实现降血糖的药物,作用机制为竞争性的抑制小肠中的α葡萄糖苷酶,从而减少淀粉分解为葡萄糖并抑制葡萄糖的吸收。由此,米格列醇应在餐前或餐中服用才会发挥该药的效果。因此,较多研究将成比例的米格列醇加入饲料中,从而模拟该药临床应用方法给药[23]。然而,本研究主要揭示其独立于延缓碳水化合物吸收之外的减重机制,侧重于对棕色脂肪细胞以及冷暴露前后棕色脂肪组织的产热激活,因此本研究未将米格列醇与饲料混合喂食小鼠,而采用脂代谢相关文献灌胃给药的方法。
本研究采用全反式维甲酸作为阳性药,由于本课题前期工作中表明其结构具有和UCP1良好对接的特点[16],且可显著增加UCP1的表达水平和脂肪产热[24],因此选择作用机制相同的化合物全反式维甲酸作为阳性药物。
本研究中,米格列醇给药对4 ℃冷暴露小鼠的体温水平显著上调,但对于−20 ℃冷暴露后的改善作用并不明显,分析其原因可能与脂肪代谢底物产热不足以抵抗长时间更低的冷环境,且UCP1产热活性尚需进一步激活。
综上,本研究明确了米格列醇对于冷暴露过程中的体温调节作用和抗足趾肿胀的寒冷损伤作用,但其如何调控UCP1的产热活性及深入机制尚需后续进一步探讨。
Anti-frostbite effect of miglitol on cold-exposed mice through UCP1-mediated thermogenic activation
doi: 10.12206/j.issn.2097-2024.202404005
- Received Date: 2024-04-02
- Rev Recd Date: 2024-07-03
- Available Online: 2025-01-16
- Publish Date: 2025-01-25
-
Key words:
- miglitol /
- brown adipose /
- thermogenesis /
- cold injury /
- UCP1
Abstract:
| Citation: | LI Xiang, LU Hongyuan, ZHANG Mingyu, GAO Huan, YAO Dong, XU Zihua. Anti-frostbite effect of miglitol on cold-exposed mice through UCP1-mediated thermogenic activation[J]. Journal of Pharmaceutical Practice and Service, 2025, 43(1): 1-5, 16. doi: 10.12206/j.issn.2097-2024.202404005 |







 DownLoad:
DownLoad: