-
肝纤维化是由肝炎病毒、酒精摄入过量或代谢紊乱引起的急性/慢性肝损伤的一种病理伤口愈合反应,也是慢性肝病发病率和病死率高的主要原因[1, 2]。肝纤维化的特点是I 型胶原和纤维连接蛋白等细胞外基质(ECM)成分的过多聚集,形成纤维疤痕扭曲肝脏结构,最终造成肝脏器官功能损伤[2]。研究显示,肝星状细胞(HSCs)的过度激活是肝纤维化进程中的关键环节,也是肝纤维化防治研究的重要靶点[3, 4]。
全反式维甲酸(ATRA)是维生素A主要的生物活性形式,已是急性早幼粒细胞白血病的标准治疗方案[5]。近期研究证实,ATRA可逆转HSCs的活化,并对肝纤维化具有抑制作用,但其具体机制尚未完全阐明[6]。本文拟在细胞水平探索ATRA抑制HSCs增殖及活化的作用和机制,为ATRA的临床应用提供理论和实验基础。
-
HSCs系LX-2细胞和培养基购自上海中乔新舟生物科技公司。胎牛血清(FBS)购自美国Gibco公司。血小板源性生长因子(PDGF-bb)、ATRA购自美国MedChemExpress公司。RNAiso试剂,逆转录和定量PCR试剂盒购自大连宝生物公司。PCR引物由上海生工生物工程公司合成。CCK-8、活性氧(ROS)、还原型谷胱甘肽(GSH)和丙二醛(MDA)等检测试剂盒购自上海碧云天生物科技公司。抗α-SMA、Collagen I、NRF2和LC3的抗体购自武汉三鹰生物科技公司;抗HO-1、ATF4、Beclin 1和GAPDH的抗兔购自武汉博士德生物科技公司。辣根过氧化物酶及FITC标记的二抗购自美国thermo Fisher Scientific公司。
-
HSCs系LX-2细胞解冻后,在DMEM培养基(含2%FBS),37 ℃,5%CO2条件下生长,每2 d更换一次培养基,细胞融合度达到85%以上时传代培养。以含PDGF-bb(10 ng/ml)的DMEM培养基作为诱导培养基;ATRA以5 μmol/L的浓度刺激。
-
细胞生长活力通过CCK-8法检测。以每孔2×103个细胞的密度将LX-2细胞接种于96孔培养板,培养过夜。分别在常规培养基(含10%FBS)和PDGF-bb诱导培养基中培养,并添加ATRA(5 μmol/L)刺激。培养目标时间后,每孔加入10 μl的CCK-8溶液,继续培养1 h,轻轻拍动培养板充分混匀后,在酶标仪上测定450 nm的吸光度(A),并绘制细胞生长活力曲线。
-
以每孔5×104个细胞的密度将LX-2细胞接种于24孔培养板(预置细胞爬片),培养过夜后以不同方式刺激培养48 h,以4%的多聚甲醛固定,并经Triton X-100(0.1%)透化10 min,随后经1%的BSA封闭1 h,加入一抗,4 ℃过夜孵育后,加入FITC标记的二抗,避光孵育1 h。加入DAPI染色1 min,以PBS-T缓冲液清洗后,用抗淬灭封片剂封片,于荧光倒置显微镜下观察并拍照。
-
以每孔2×105个细胞的密度将LX-2细胞接种于12孔培养板,培养过夜后以不同方式刺激培养48 h,利用RIPA试剂提取总蛋白。以20 μg总蛋白作为上样量,经SDS凝胶电泳分离后,转至甲醇预处理的PVDF膜。以5%脱脂牛奶常温封闭1 h,加入一抗,4 ℃过夜孵育后,加入辣根过氧化物酶标记二抗,常温孵育1 h。用TBS-T缓冲液清洗3次,经显色后在凝胶成像仪上观察并拍照记录。
-
以每孔2×105个细胞的密度将LX-2细胞接种于12孔培养板,培养过夜后以不同方式刺激培养48 h,利用trizol试剂提取总RNA。以200 ng总RNA为模板,逆转录成互补DNA(cDNA),反应程序为37 ℃,10 min,85 ℃,5 s。以稀释后的cDNA为模板进行实时定量PCR反应,反应程序为95 ℃,15 s;56 ℃,20 s;72 ℃,20 s,共40个循环。以GAPDH基因作为内参,每个样品重复3次,经2−△△Ct法计算目的基因的相对表达水平。
-
细胞内ROS利用DCFH-DA荧光探针检测。以每孔1×104个细胞的密度将LX-2细胞接种于24孔培养板,培养过夜后以不同方式刺激培养48 h。去除培养液并加入100 μl含DCFH-DA(10 μmol/L)的无血清培养基,继续孵育20 min。用无血清培养基清洗3次后,在荧光显微镜下观察并拍照记录。
以每孔1×105个细胞的密度将LX-2细胞接种于6孔培养板,培养过夜后以不同方式刺激培养48 h。细胞内GSH和MDA水平根据试剂盒说明书进行检测,计算总蛋白中GSH和MDA的含量(nmol/mg)。
-
双荧光自噬流通过转染自噬双标腺病毒(pAd-mRFP-GFP-LC3)后检测。以每孔1×104个细胞的密度将LX-2细胞接种于24孔培养板,培养过夜。将腺病毒转染细胞后,以不同方式刺激培养48 h,即在荧光显微镜下分别观察红色及绿色荧光信号并拍照记录。自噬激活后,自噬体与溶酶体融合后绿色荧光发生淬灭,红色荧光增强。
-
所有数据均使用SPSS 26.0软件进行统计学分析,满足正态分布的计量数据以(
$ \bar x \pm s $ )表示。组间差异以独立样本t检验比较分析,以P<0.05说明差异具有统计学意义。 -
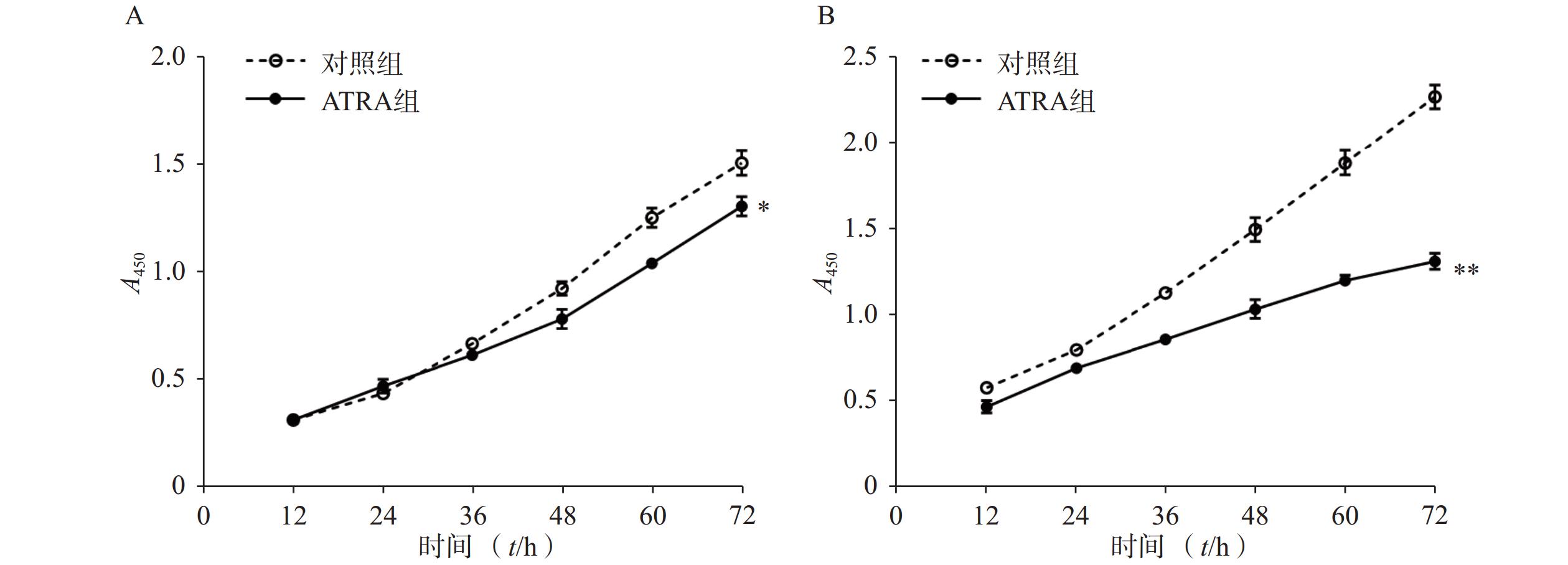
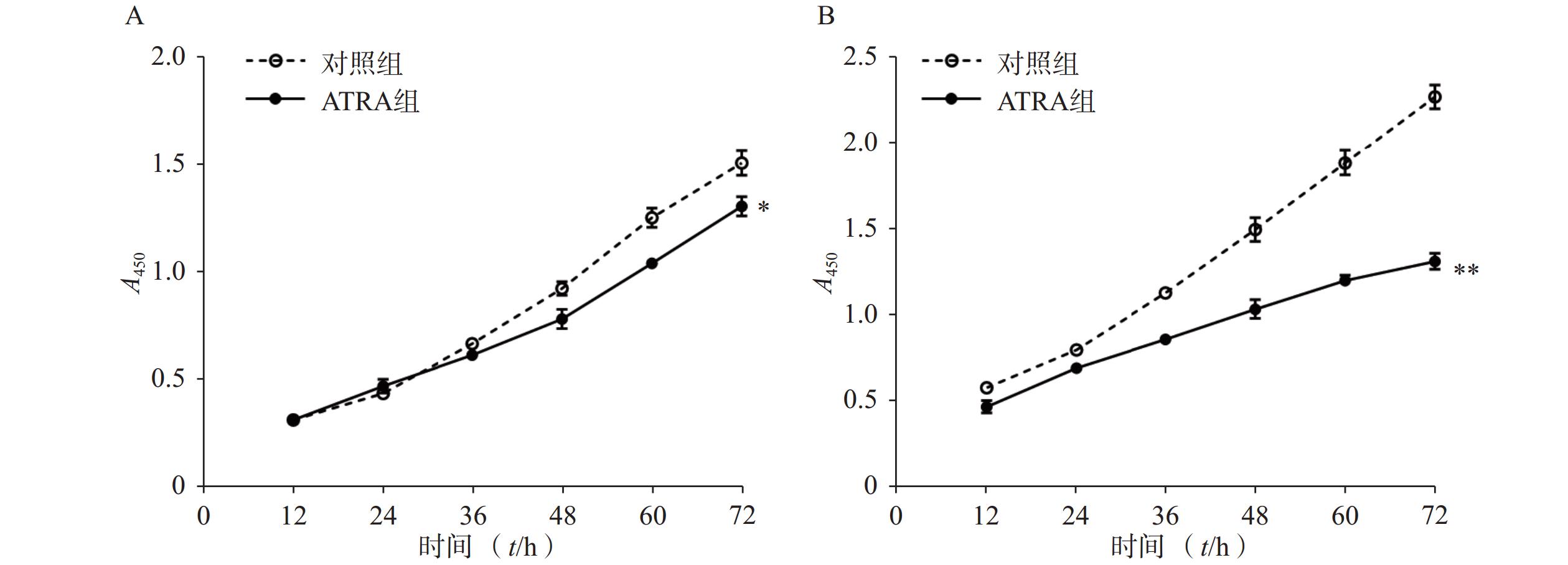
如图1A所示,常规培养条件下,5 μmol/L的ATRA处理48 h和72 h后的HSCs生长活力为对照组的(84.5±4.8)%和(86.7±3.0)%,具有一定的抑制作用。如图1B所示,在PDGF-bb诱导条件下,5 μmol/L的ATRA处理48 h和72 h后的HSCs生长活力为对照组的(52.4±3.0)%和(57.6±2.0)%,具有显著的抑制作用(P<0.01)。
-
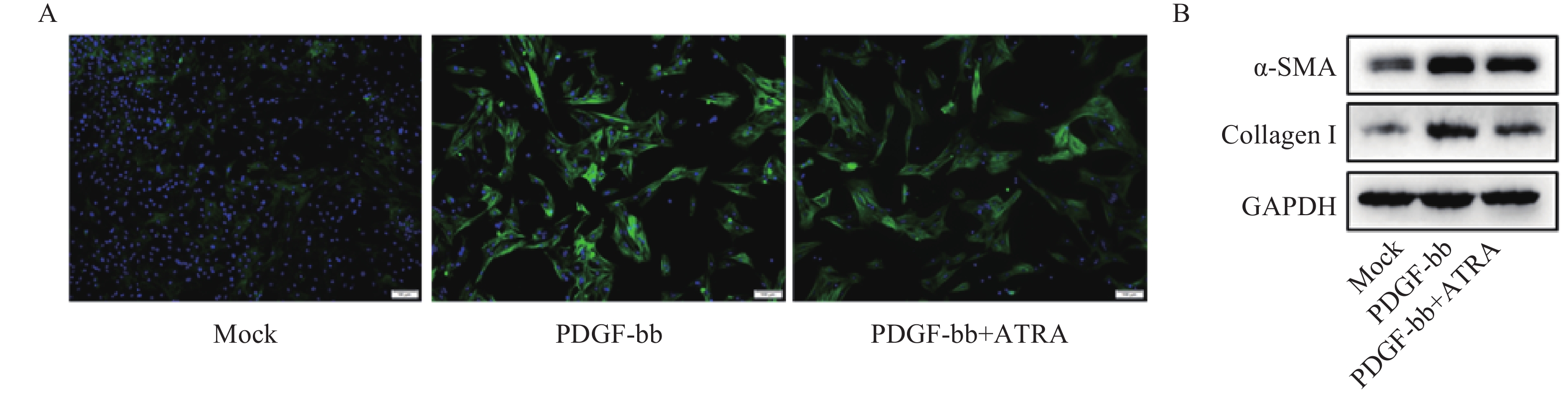
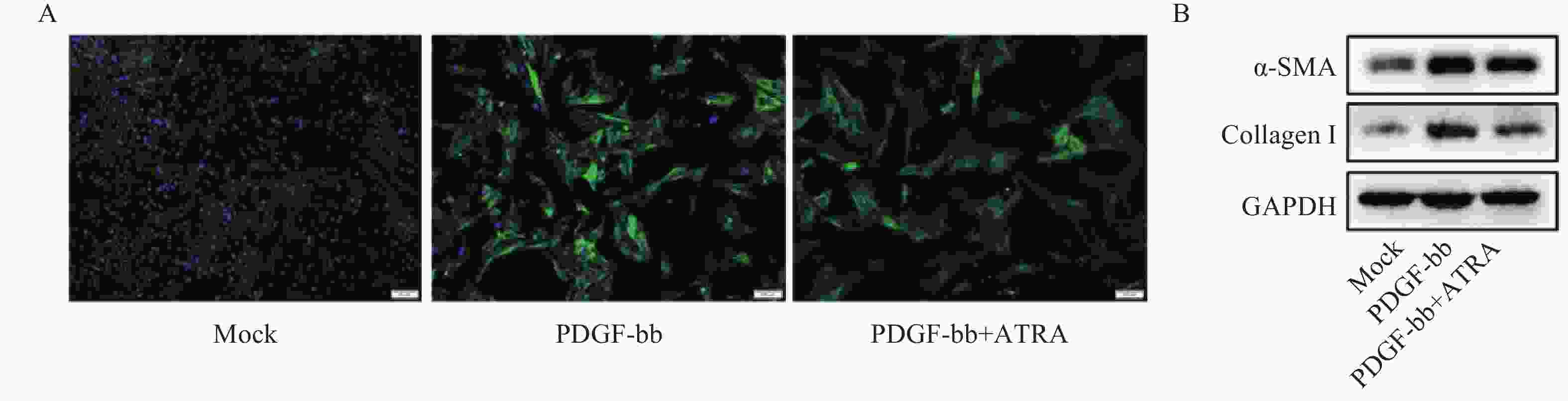
免疫荧光结果如图2A所示,与对照组相比,PDGF-bb刺激的HSCs中α-SMA的绿色荧光信号较强,而ATRA处理后,α-SMA荧光信号显著降低。蛋白质免疫印迹的结果如图2B所示,与对照组相比,PDGF-bb刺激的HSCs中α-SMA和Collagen I的蛋白表达明显增加,而ATRA处理后,α-SMA和Collagen I蛋白表达明显降低。
-
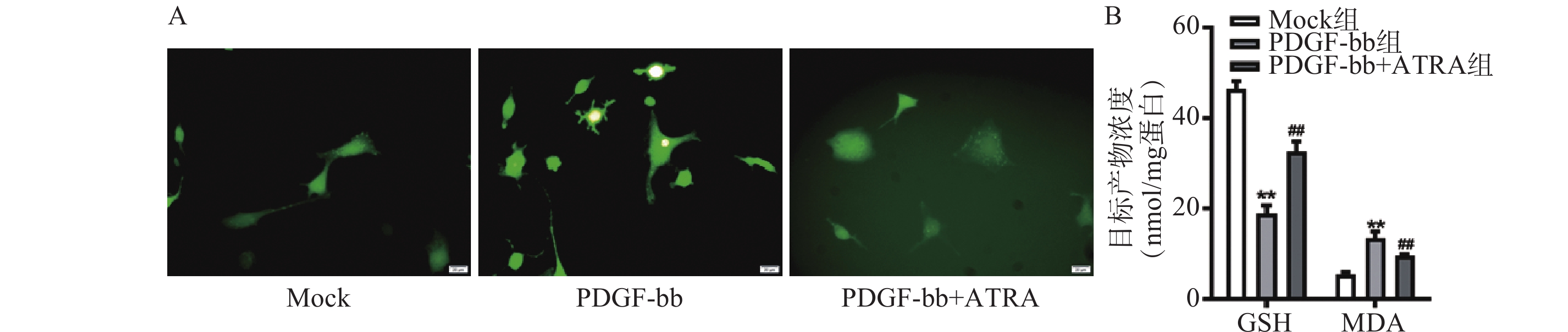
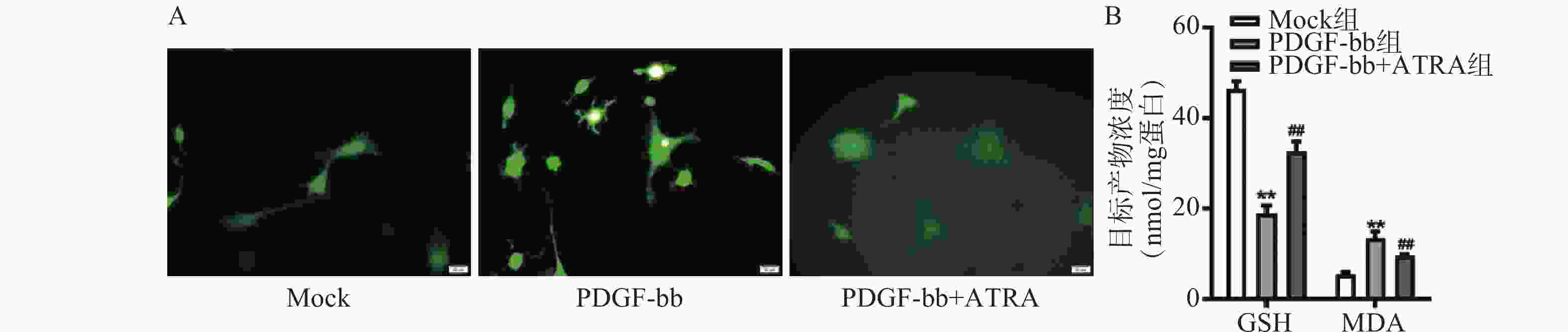
ROS的检测如图3A所示,PDGF-bb刺激后HSCs中的荧光强度明显强于对照组,而ATRA处理后荧光强度明显降低,提示ATRA抑制HSCs中ROS的生成。如图3B所示,PDGF-bb刺激后,HSCs中GSH的含量明显低于对照组(18.82±1.83 nmol/mg vs. 46.45±1.69 nmol/mg),MDA的含量则明显高于对照组(13.46±1.43 nmol/mg vs. 5.45±0.47 nmol/mg);ATRA处理后GSH的含量明显升高(32.60±2.23)nmol/mg,MDA的含量则明显降低(9.56±0.34)nmol/mg。
-
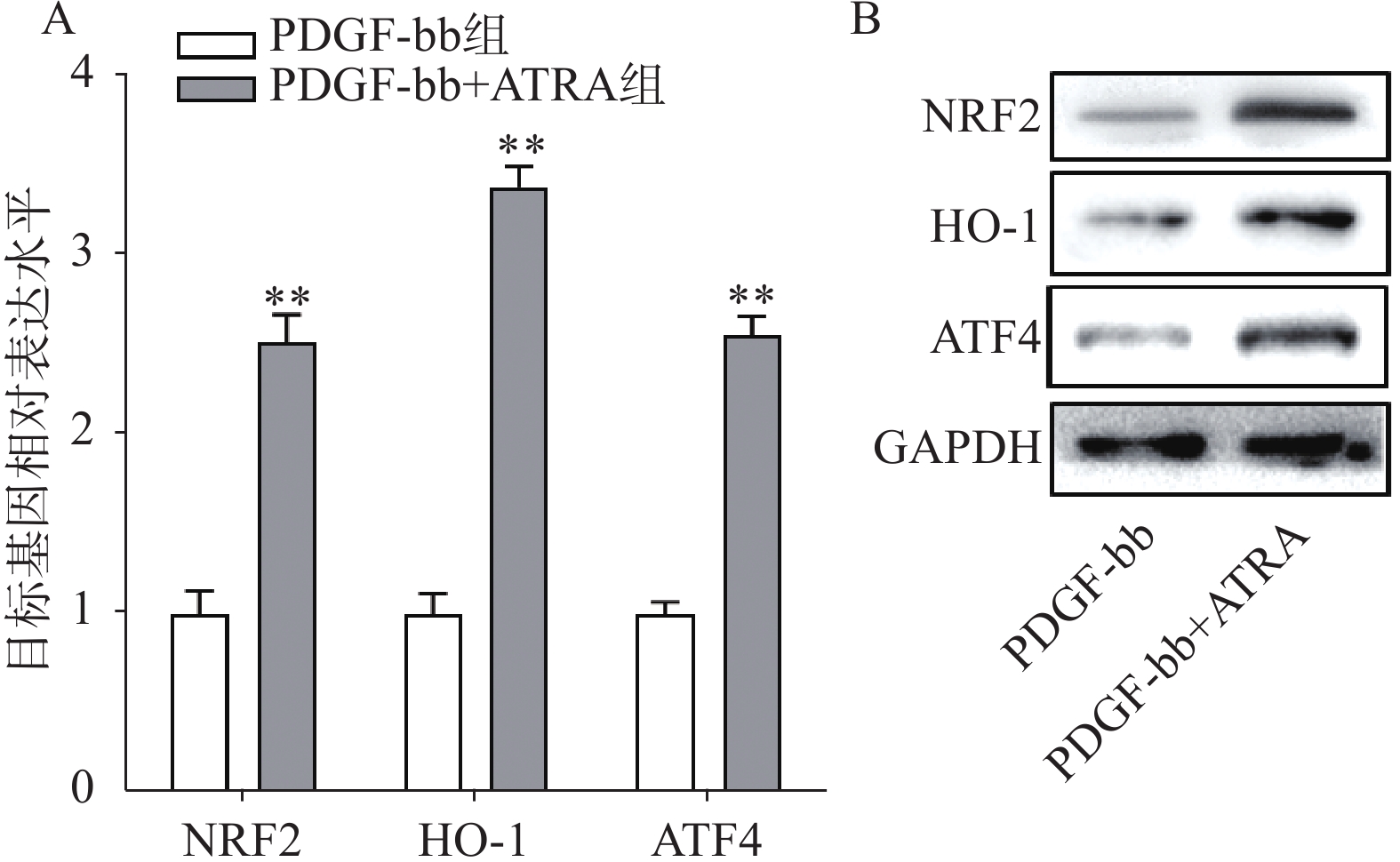
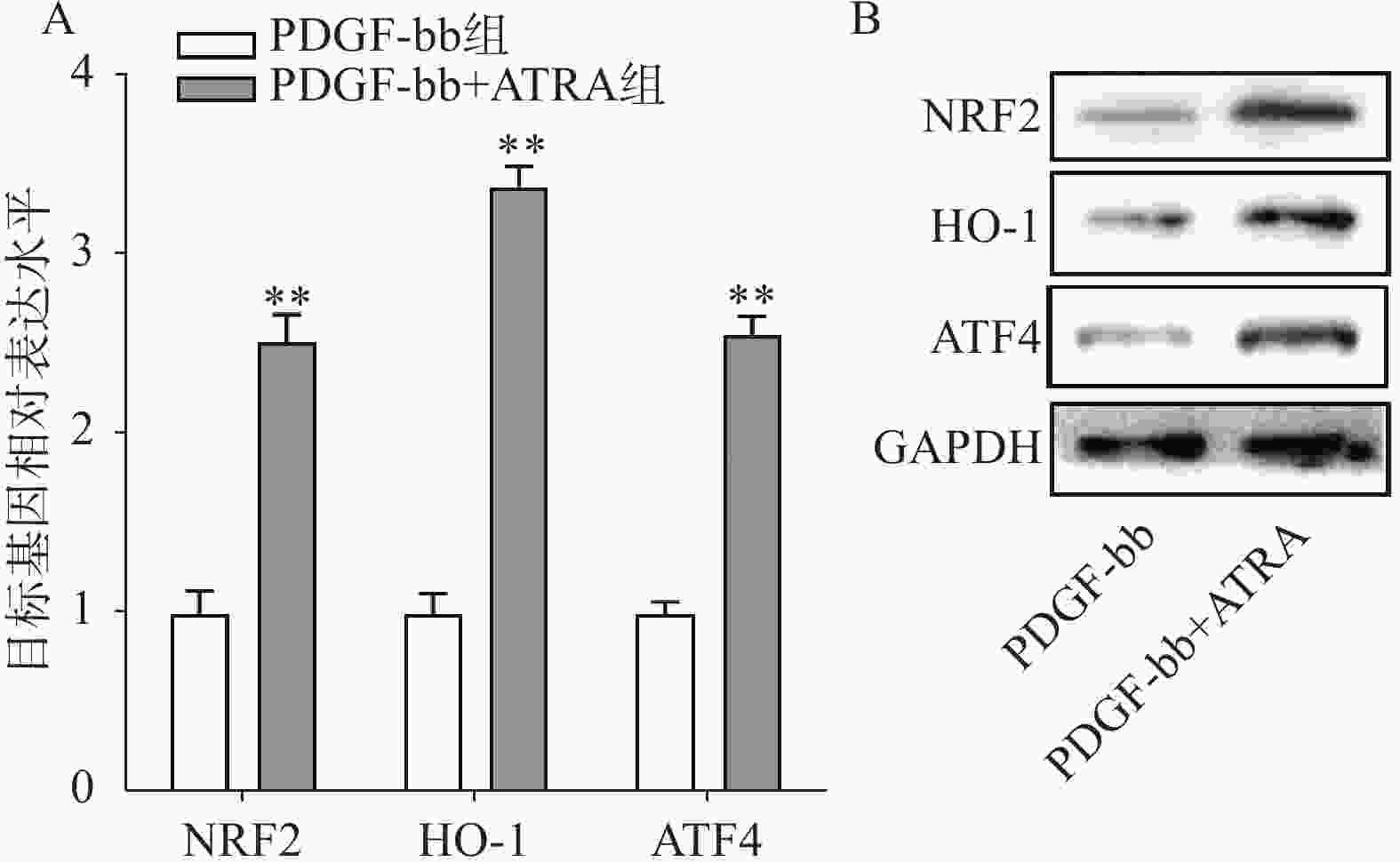
实时定量PCR的检测结果如图4A所示,ATRA处理后HSCs中抗氧化基因NRF2、HO-1和ATF4的表达明显增加(P<0.01),分别是PDGF-bb组的(2.53±0.15)倍、(3.34±0.12)倍和(2.58±0.10)倍。蛋白质免疫印迹的结果如图4B所示,NRF2、HO-1和ATF4的蛋白表达在ATRA处理后也明显增加。
-
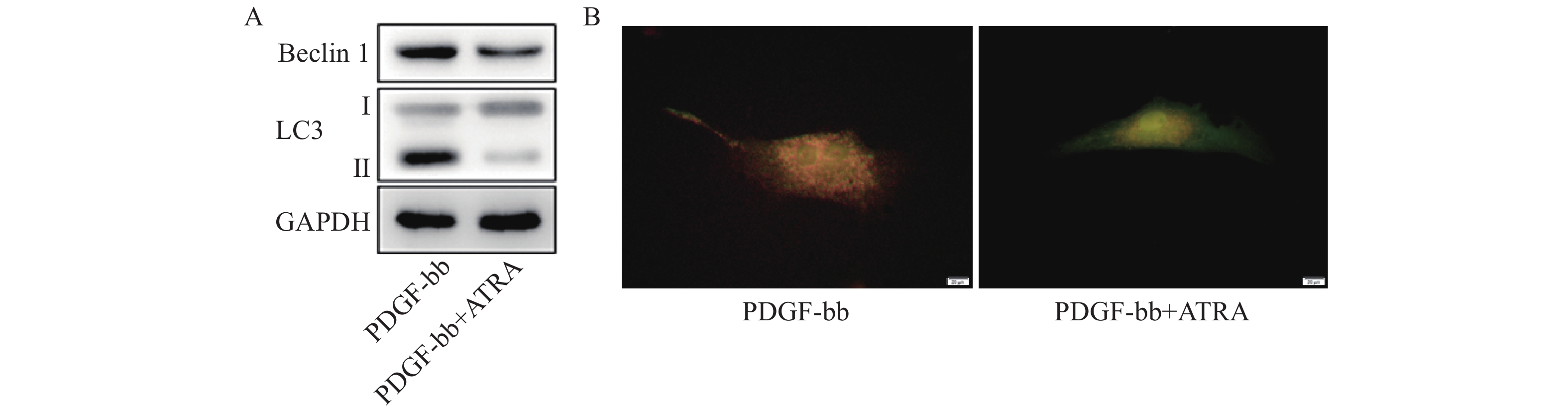
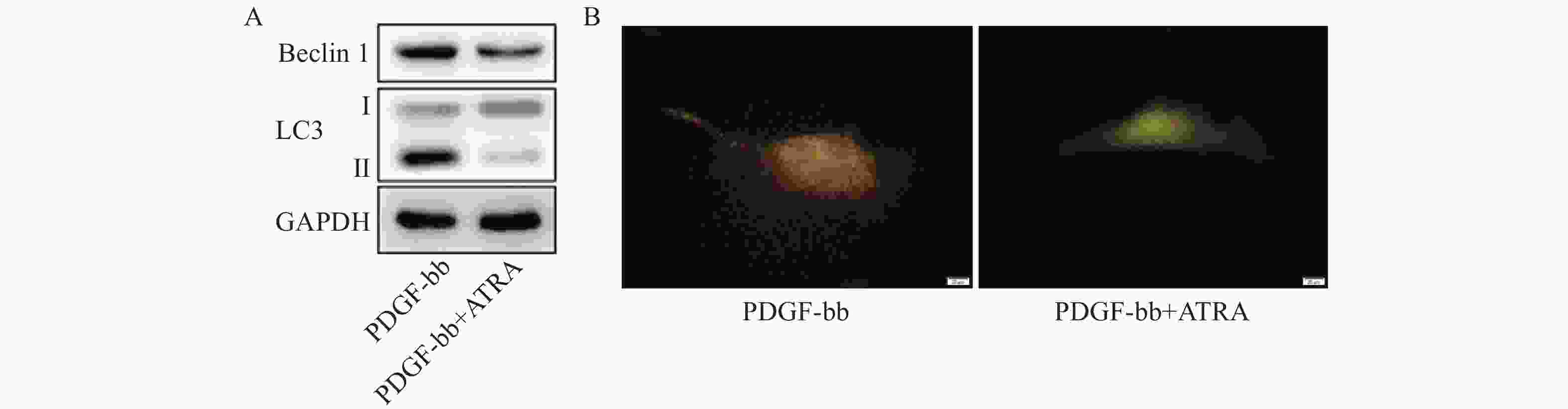
蛋白质免疫印迹的结果如图5A所示,ATRA处理后HSCs中自噬标志蛋白Beclin 1的表达和LC3 II/I均明显减少。双荧光自噬流的检测结果如图5B所示,ATRA处理后HSCs中红色荧光信号显著降低,绿色荧光信号明显增强,自噬流信号显著降低。
-
目前,还没有特定的抗纤维化疗法来预防或逆转肝纤维化,现有的治疗方案旨在去除潜在的致病因子或紊乱,但也证明了肝纤维化的潜在可逆性[7]。研究证实,HSCs是主要的肝胶原生成细胞,并被认为是肝纤维化的主要效应细胞[8]。在健康肝脏中,HSCs位于窦周间隙,处于静止状态;当受到损伤或刺激时,HSCs会对各种促纤维化信号做出反应,如来自受损肝细胞的产物、来自Kupffer细胞的生长因子和细胞因子、重构的ECM以及肝外信号等[9]。HSCs的活化涉及多个进程,包括细胞死亡、衰老和恢复静止状态等。在此过程中,HSCs会失去其特有的细胞质脂滴,并转分化为肌成纤维样细胞,并合成ECM成分(如胶原纤维I型和III型),增殖、收缩和迁移等能力增强,并具有促炎作用。HSCs活化的作用机制和干预是肝纤维化防治研究的重要内容。
静息状态HSCs中富含的脂滴,其主要成分是甘油三酯和维生素A。脂滴脱落被认为是HSCs活化的形态学标志,但其生物学作用仍被广泛研究[10-12],作为维生素A的主要代谢产物,ATRA被发现广泛参与肝纤维化以及肝癌等的生物学过程[13, 14]。研究发现,ATRA能通过抑制硫氧还蛋白互用蛋白的表达,改善TGF-β诱导的HSCs的活化和维生素A缺乏诱导的肝纤维化[6]。目前的研究证实,ATRA 通过与 RAR(α、β、γ)或 PPARβ/δ 起作用,但这两组受体的生物效应在某些进程中却完全相反[15],提示ATRA抗肝纤维化的作用机制未完全阐明。还有研究发现,纤维化药物对HSCs的激活最终会导致这些细胞的衰老,尽管有助于纤维化的逆转,但也可能会导致肝癌的发生[16]。因此,ATRA作为抗肝纤维化潜在药物的应用潜能仍需深入探索和验证。
研究证实,氧化应激与肝纤维化之间存在密切关系,当肝脏受到氧自由基的攻击时,机体抗氧化防御系统的平衡就会被打破,从而导致氧化应激[17, 18]。MDA是脂质过氧化的主要代谢产物,过量时会严重破坏细胞膜结构,也被认为是肝脏中自由基的间接指标[19]。GSH是哺乳动物细胞中最主要的自由基清除剂,广泛分布于包括肝脏在内的多个器官。研究发现,GSH可保护肝细胞免受各种自由基的侵害,包括ROS、脂质氢过氧化物、异生物毒物和重金属[20]。因此,调节MDA和GSH水平有助于控制氧化应激,最终可能有助于抑制肝纤维化。有研究报道,抑制氧化应激显著降低MDA水平,提高GSH水平,对CCl4诱导的小鼠肝脏纤维化具有明显的保护作用[21]。本课题研究发现ATRA处理促进了HSCs中抗氧化基因的表达,造成细胞中GSH的增加和MDA的降低,减少细胞内ROS的水平,缓解了PDGF-bb诱导的HSCs氧化应激,研究结果提示,以氧化应激为靶点可能是肝纤维化防治的潜在策略。
自噬是真核细胞消除一次性或有潜在危险的细胞质物质的一种新陈代谢过程,可以消除有缺陷的蛋白质和细胞器、细胞内的病原体,防止异常蛋白质的积累,在许多疾病的病理过程中发挥着积极作用[22]。越来越多的证据表明,肝细胞和非实质性细胞(HSCs、Kupffer 细胞等)的自噬反应对肝脏的生理功能至关重要[23]。近年来,HSCs自噬成为肝纤维化研究领域的热点,其调控机制日益受到关注。研究显示,自噬水平的增加可加速HSCs中脂滴的降解,为HSCs的激活提供能量支持[24, 25]。降低HSCs自噬活性显著抑制其活化,降低小鼠的肝纤维化程度[26]。本课题的结果证实,ATRA能抵抗PDGF-bb诱导的HSCs自噬水平的增加,这可能是ATRA抑制HSCs活化的分子机制之一。
HSCs是目前研究药物代谢和毒理的重要工具,也是研究肝纤维化的绝佳模型[27]。HSCs具有高度的可塑性,在有害刺激下会改变其表型和代谢,并产生大量ECM成分来替代受伤细胞,生成纤维化疤痕[28]。在各种HSCs细胞系中,LX-2是目前广泛使用的细胞类型[29]。研究发现,LX-2细胞会根据培养基中FBS的浓度改变其代谢,从而诱导基因的差异表达。在低浓度FBS下,LX-2细胞呈静止样表型;在高浓度 FBS下,细胞呈肌成纤维细胞样表型,产生ECM成分[30]。本课题研究发现,PDGF-bb可诱导LX-2细胞向肌成纤维细胞样表型分化,表现为高增殖水平,且高表达α-SMA和Collagen I,而ATRA的处理显著降低细胞增殖能力并抑制α-SMA和Collagen I的表达,表明ATRA具有抵抗HSCs活化的潜在功能。
本课题在细胞水平探索了ATRA对HSCs激活的作用和潜在机制。ATRA促进抗氧化基因的表达,降低PDGF-bb诱导的HSCs氧化应激及自噬活力,抑制了HSCs的激活。研究结果证实了ATRA对肝纤维化防治的潜在应用。需要注意的是,自噬活性的激活存在“双刃剑”作用,ATRA对HSCs自噬活性的调节还需要进行剂量和分子机制等方面的深入研究。
Exploration of the role and mechanism of all-trans retinoic acid on activation and oxidative stress of hepatic stellate cell
doi: 10.12206/j.issn.2097-2024.202312054
- Received Date: 2023-12-21
- Rev Recd Date: 2024-05-09
- Available Online: 2024-07-16
- Publish Date: 2024-07-25
-
Key words:
- all-trans retinoic acid /
- hepatic stellate cells /
- activation /
- oxidative stress /
- autophagy
Abstract:
| Citation: | XIU Jianping, YANG Chaoai, LIU Xi’ao, PAN Qianyu, WEI Guangxu, WANG Weixing. Exploration of the role and mechanism of all-trans retinoic acid on activation and oxidative stress of hepatic stellate cell[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(7): 291-296. doi: 10.12206/j.issn.2097-2024.202312054 |








 DownLoad:
DownLoad: