-
肺癌是一种常见的恶性肿瘤,其中,非小细胞肺癌(NSCLC)占肺癌的80%~85%,在全球范围内的发病率和病死率显著较高[1-2]。2020年全球估计有220万新发肺癌病例(占全球癌症病例总数的11.4%)和近180万肺癌死亡病例(占全球癌症死亡总数的18.0%)。而中国肺癌病例数和病死率(分别为37.0%和39.8%)为全球肺癌发病之首[3-4]。
传统的NSCLC治疗方式主要包括化疗、手术切除、放疗和最佳的支持性治疗。然而,化疗只能提供适度的好处,而安全性有限,因此最近几年上市的免疫检查点抑制剂(ICIs)已成为NSCLC的主要的治疗方法[5],特别是程序性死亡受体-1 (PD-1)和抗程序性死亡配体-1 (PD-L1)的免疫检查点抑制剂,通过恢复被抑制的效应T细胞的功能来增强抗肿瘤作用,并在大量转移性和晚期NSCLC患者中产生持久的反应[6]。
纳武利尤单抗(nivolumab)是一种全人源性的IgG4 PD-1免疫检查点抑制剂抗体,于2015年获得了美国FDA的批准,用于治疗NSCLC患者,在美国国立综合癌症网络(NCCN)的指南中,纳武利尤单抗被推荐为治疗NSCLC的一线药物。此药于2019年在国内上市,获批用于含铂类方案化疗后疾病进展或不可耐受的局部晚期或转移性NSCLC成人患者。但由于其上市时间较短,临床上应用有效性和安全性数据匮乏,本研究纳入最新的8项Ⅲ期随机对照临床试验(RCTs),共有4 945例患者参与。旨在通过Meta分析的方法评估纳武利尤单抗治疗NSCLC的有效性和安全性。
-
计算机检索PubMed、Embase、Cochrane Library、中国知网(CNKI)、维普中文科技期刊数据库、万方医学数据库中有关纳武利尤单抗治疗NSCLC的相关研究,检索时间自建库至2023年3月。英文检索词为“Nivolumab” “Opdivo” “ONO-4538” “MDX-1106” “BMS-936558” “Non-Small-Cell Lung Carcinomas” “Non-Small Cell Lung Cancer” “NSCLC” “Carcinoma” “Non-Small-Cell Lung”等;中文检索词为:“非小细胞肺癌”“纳武利尤单抗”“欧狄沃”“纳武单抗”等,不限制试验类型。
-
纳入标准:①基于Ⅲ期RCTs的研究。②研究对象为经组织学或细胞学证实为处于Ⅲ-Ⅳ期的NSCLC患者。③干预措施:试验组患者接受纳武利尤单抗单药治疗或纳武利尤单抗单药联合传统化疗方案进行治疗,而传统化疗组患者采用传统化疗方案或安慰药联合传统化疗方案进行治疗。④研究结果包括总生存期(OS)、无进展生存期(PFS)及三级以上不良事件的发生(如恶心、腹泻、中性粒细胞减少、皮疹、贫血、食欲减退、疲劳)。
不符合纳入标准的研究和共享相同数据集的研究被排除在外。如果为同一临床试验发表了两篇以上的文章,则从每一篇文章中获取每个研究结果的最新数据。
-
两位作者使用标准化表格独立完成了数据提取,并由第三作者进行验证。所有研究数据均以标准化形式提取,一式两份,如发生争议,则由研究小组协助解决。提取的数据内容包括本研究的基本特征:第一作者和发表年份、实验阶段与类型、病理分型、样本量及样本基本信息、干预措施、结局指标、不良反应例数等。
-
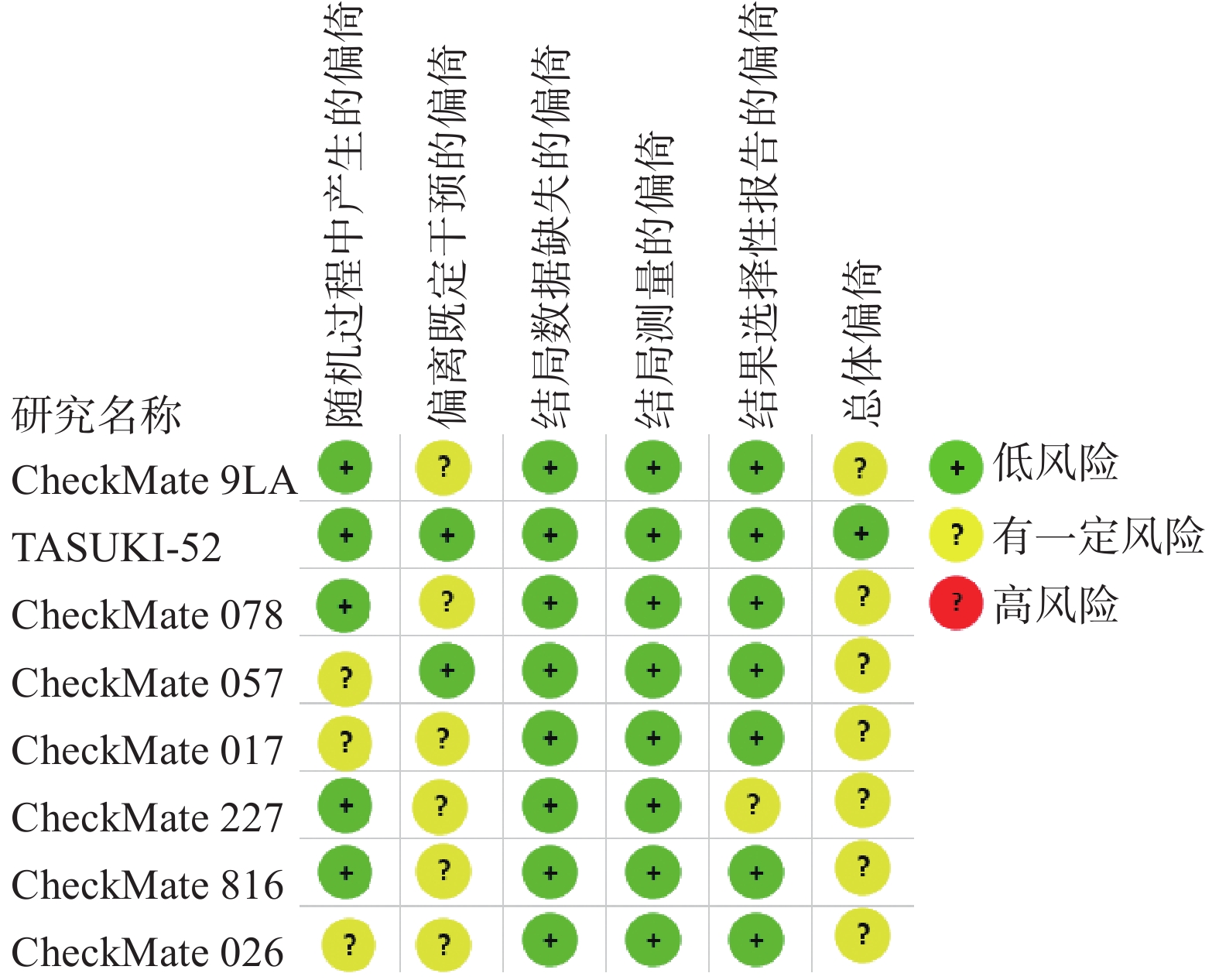
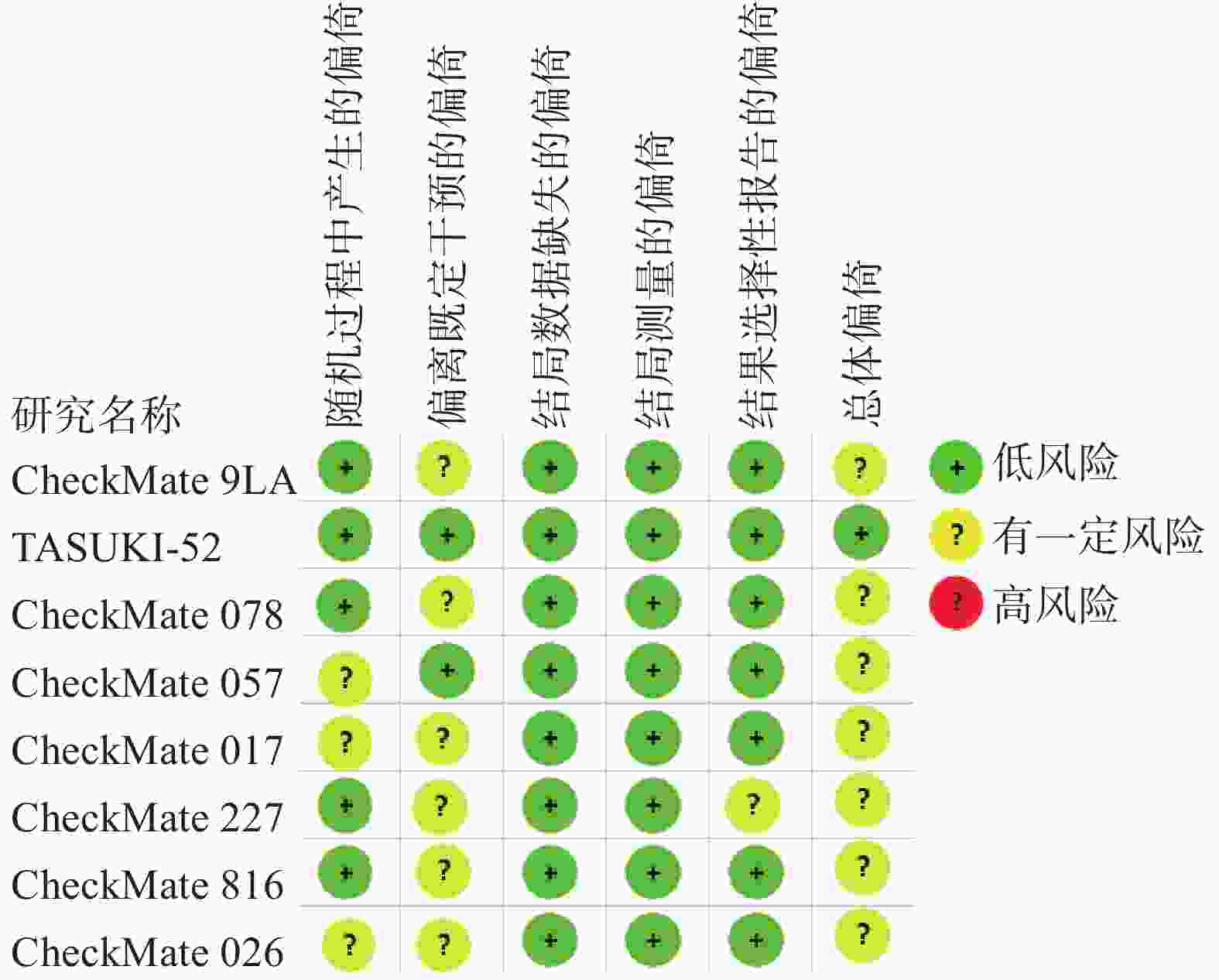
采用Cochrane偏倚评估工具2.0版本(RoB2.0)对本研究所纳入的RCTs进行质量评价,评估主要分为5个模块包括:随机过程中产生的偏倚、偏离既定干预的偏倚、结局数据缺失的偏倚、结局测量的偏倚以及结果选择性报告的偏倚。这些模块从不同角度对RCTs产生偏倚的可能性进行评估,根据工具算法,总体偏倚风险判断是“低风险”、“有一定风险”和“高风险”。
-
使用STATA13.1对提取的数据进行统计分析。对OS和PFS采用风险比(HR),不良反应采用比值比(OR)及其95%CI合并分析分类变量。OS和PFS采用HR分析(HR>1有利于对照组,HR<1有利于纳武利尤单抗组);不良反应发生率采用OR分析;采用卡方检验评估研究的异质性,当P≥0.1且I2≤50%时,采用固定效应模型;当P<0.1或I2>50%时,采用随机效应模型。
-
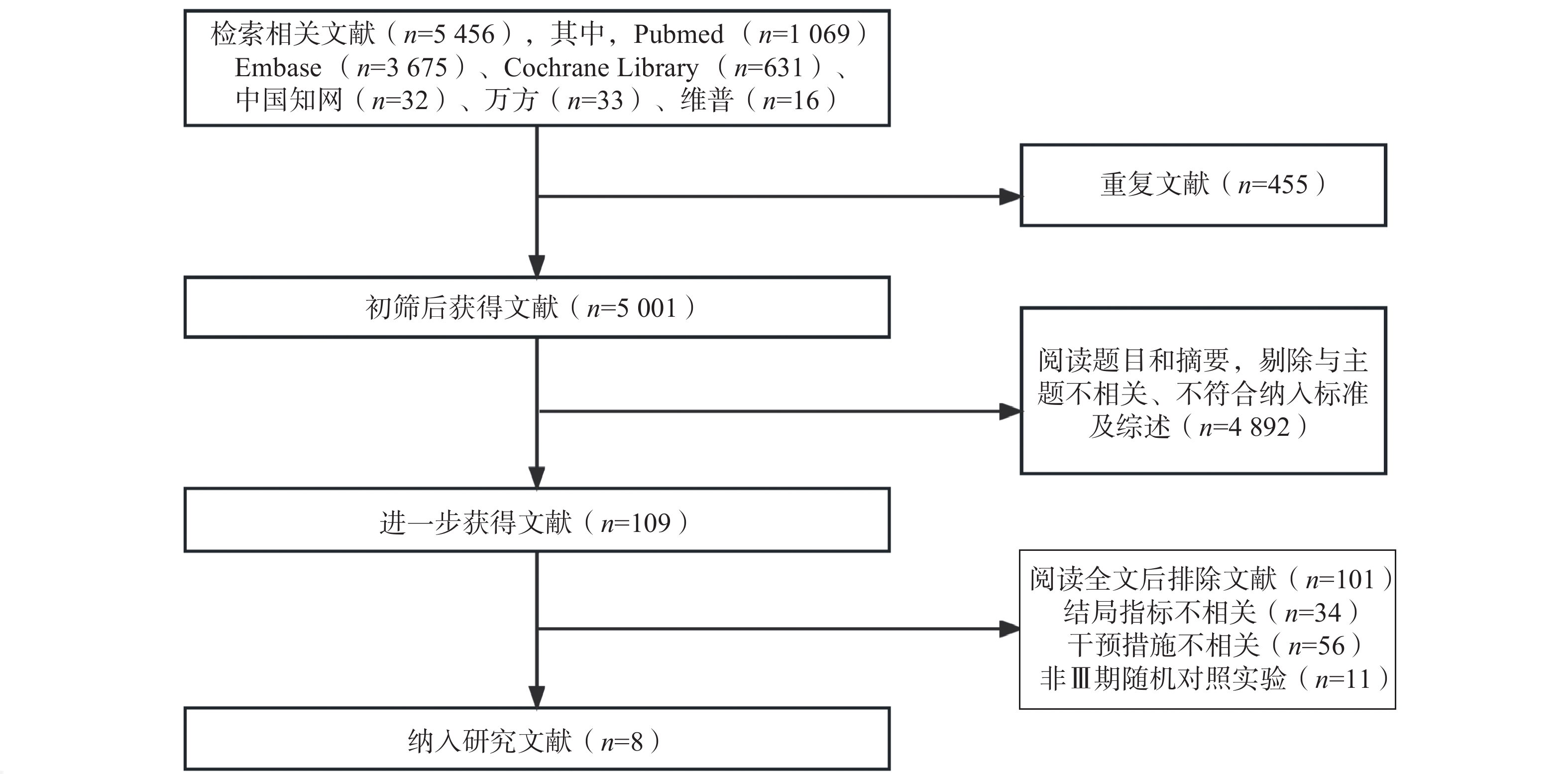
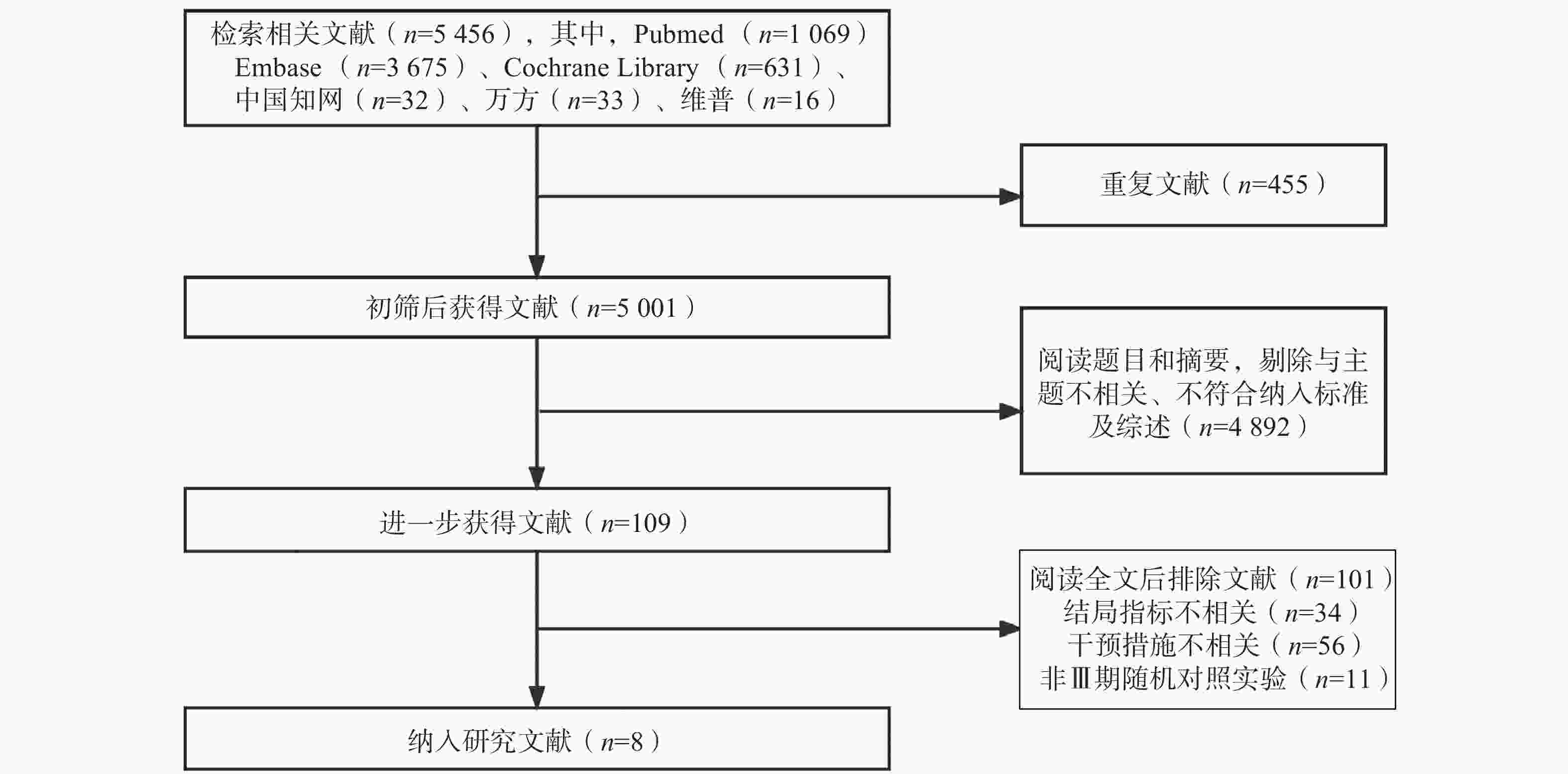
共检索相关文献5 456篇,根据纳排标准进行筛选后,最终纳入了来自8项[7-14]Ⅲ期RCTs的4 945例NSCLC患者进行统计分析。纳入研究基本信息见表1,文献筛选流程见图1。
研究 干预措施 患者例数 HR(95%CI) 相关不良反应* 试验组 对照组 试验组 对照组 总生存期 无进展生存期 CheckMate 9LA[7] 伊普利单抗+纳武利尤单抗+铂类化疗 以铂类为基础的
双重化疗361 358 0.66(0.55-0.80) 0.70(0.57-0.86) ①②③④⑤⑥⑦⑧ TASUKI-52[8] 卡铂+紫杉醇+贝伐珠单抗+纳武利尤单抗 卡铂+紫杉醇+
贝伐珠单抗+安慰剂275 275 0.85(0.66-1.10) 0.56(0.49-0.64) ①②③④⑤⑥⑦⑧ CheckMate 078[9] 纳武利尤单抗 多西他赛 338 166 0.68(0.52-0.90) 0.77(0.62-0.95) ①④⑤⑥⑦⑧ CheckMate 057[10] 纳武利尤单抗 多西他赛 292 290 0.75(0.62-0.91) 0.92(0.77-1.11) ①②④⑥⑦⑧ CheckMate 017[11] 纳武利尤单抗 多西他赛 135 137 0.59(0.44-0.79) 0.62(0.47-0.81) ①②③④⑤⑥⑦⑧ CheckMate 026[12] 纳武利尤单抗 以铂类为基础的化疗 211 212 1.02(0.80-1.30) 1.15(0.91-1.45) ①②③④⑤⑥⑦⑧ CheckMate 227[13] 伊普利单抗+
纳武利尤单抗以铂类为基础的化疗 576 570 0.73(0.64-0.84) 0.79(0.69-0.91) ①②③④⑤⑥⑦⑧ CheckMate 816[14] 纳武利尤单抗+
铂类双重化疗单独铂类双重化疗 179 179 0.57(0.38-0.87) 0.54(0.37-0.80) ①②③④⑤⑥⑦⑧ 注:* ①三级以上不良反应,②恶心,③腹泻,④中性粒细胞减少,⑤皮疹,⑥贫血,⑦食欲下降,⑧疲劳。所有研究均为经组织学或细胞学证实为处于Ⅲ-Ⅳ期的NSCLC患者。 -
对8篇RCTs采用RoB2.0工具进行评价,偏倚风险评价结果见图2。
-
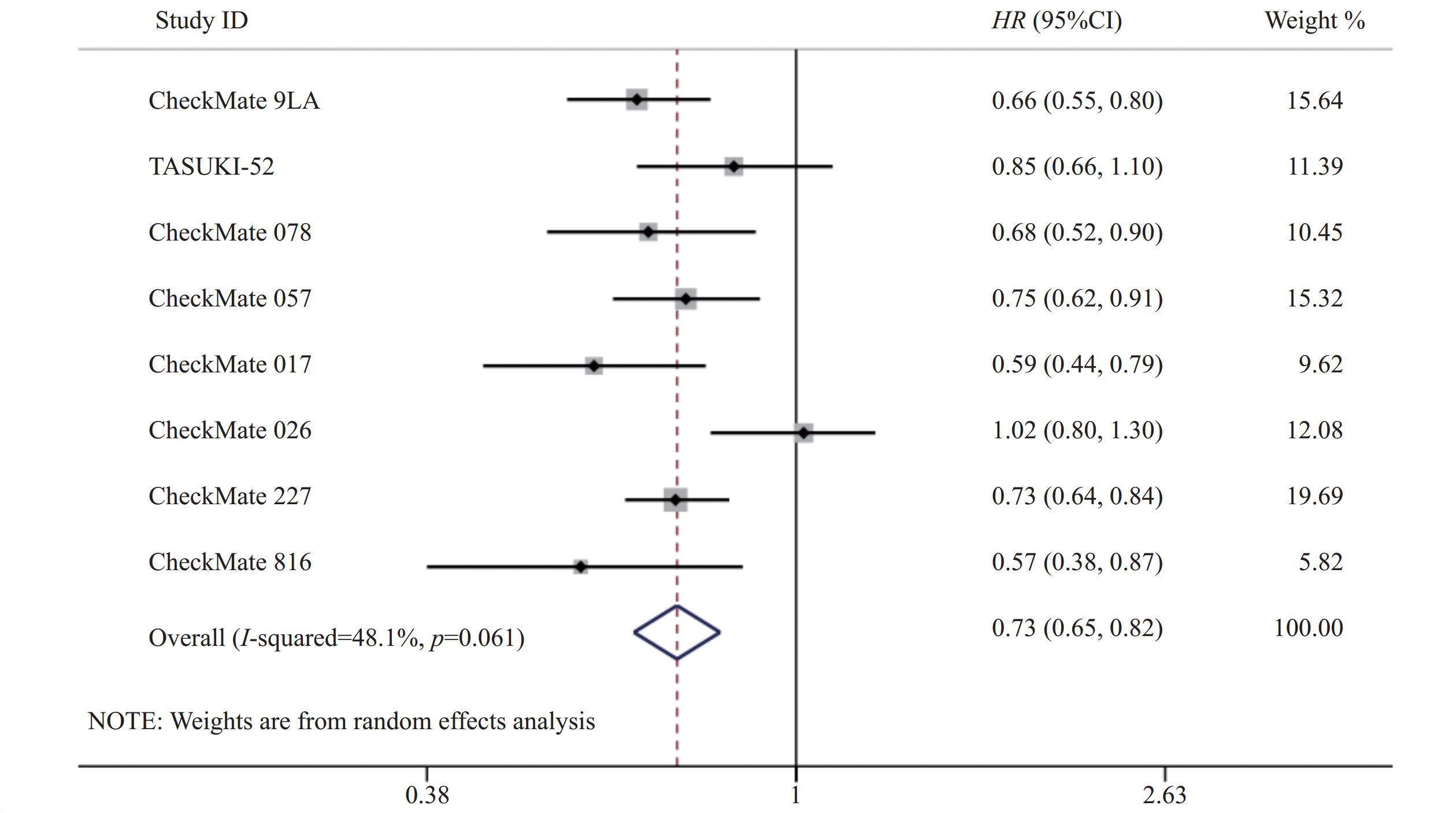
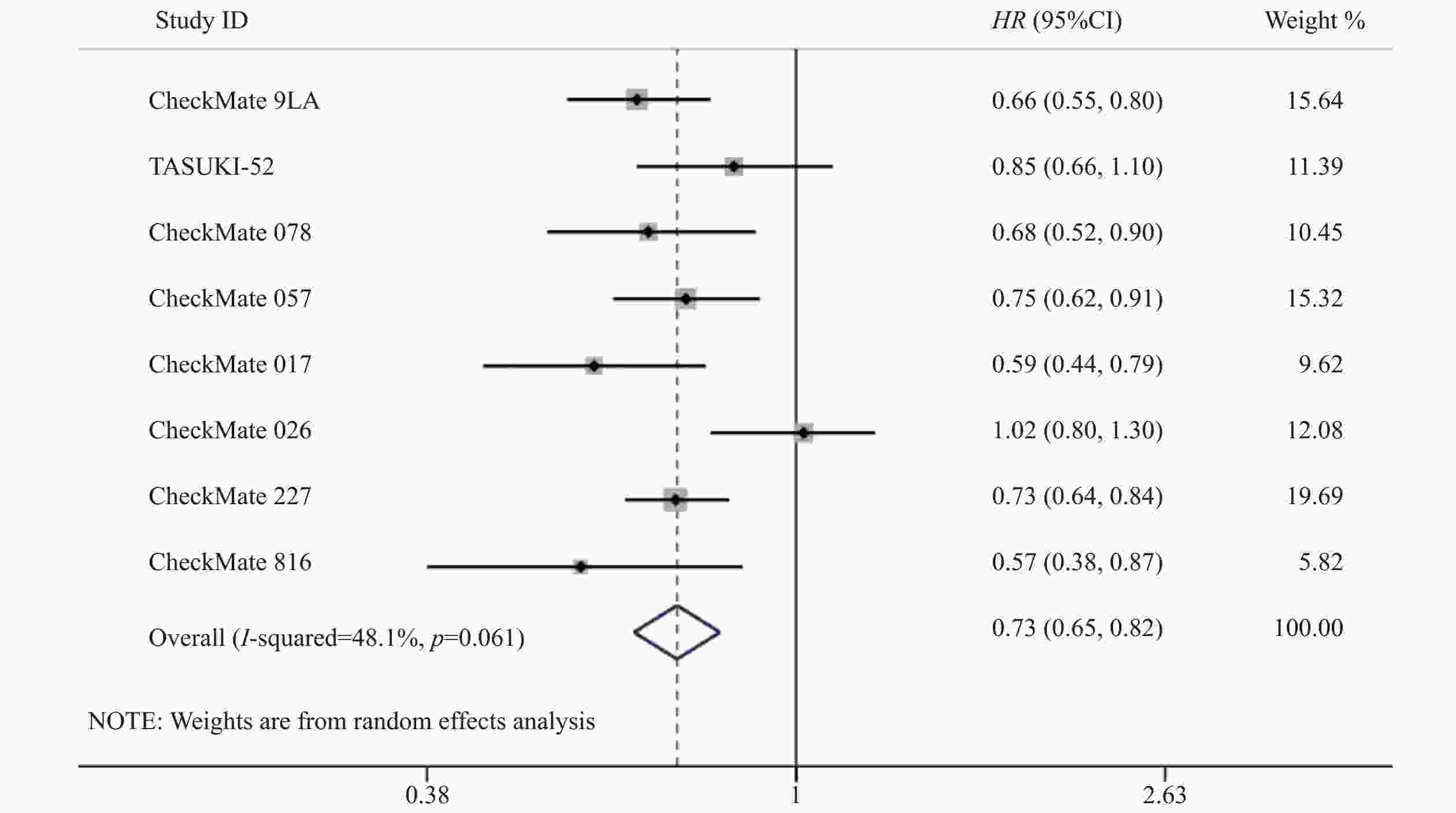
8项研究[7-14]均对OS进行了统计分析。各研究间存在统计学异质性(P=0.06,I2=48.1%),使用随机效应模型,Meta分析结果显示,与对照组相比,试验组患者接受纳武利尤单抗治疗延长了NSCLC患者的OS,差异有统计学意义(HR=0.73,95%CI=0.65~0.82,P<0.05),提示使用纳武利尤单抗治疗NSCLC的疗效要优于传统的化疗方案,见图3。
-
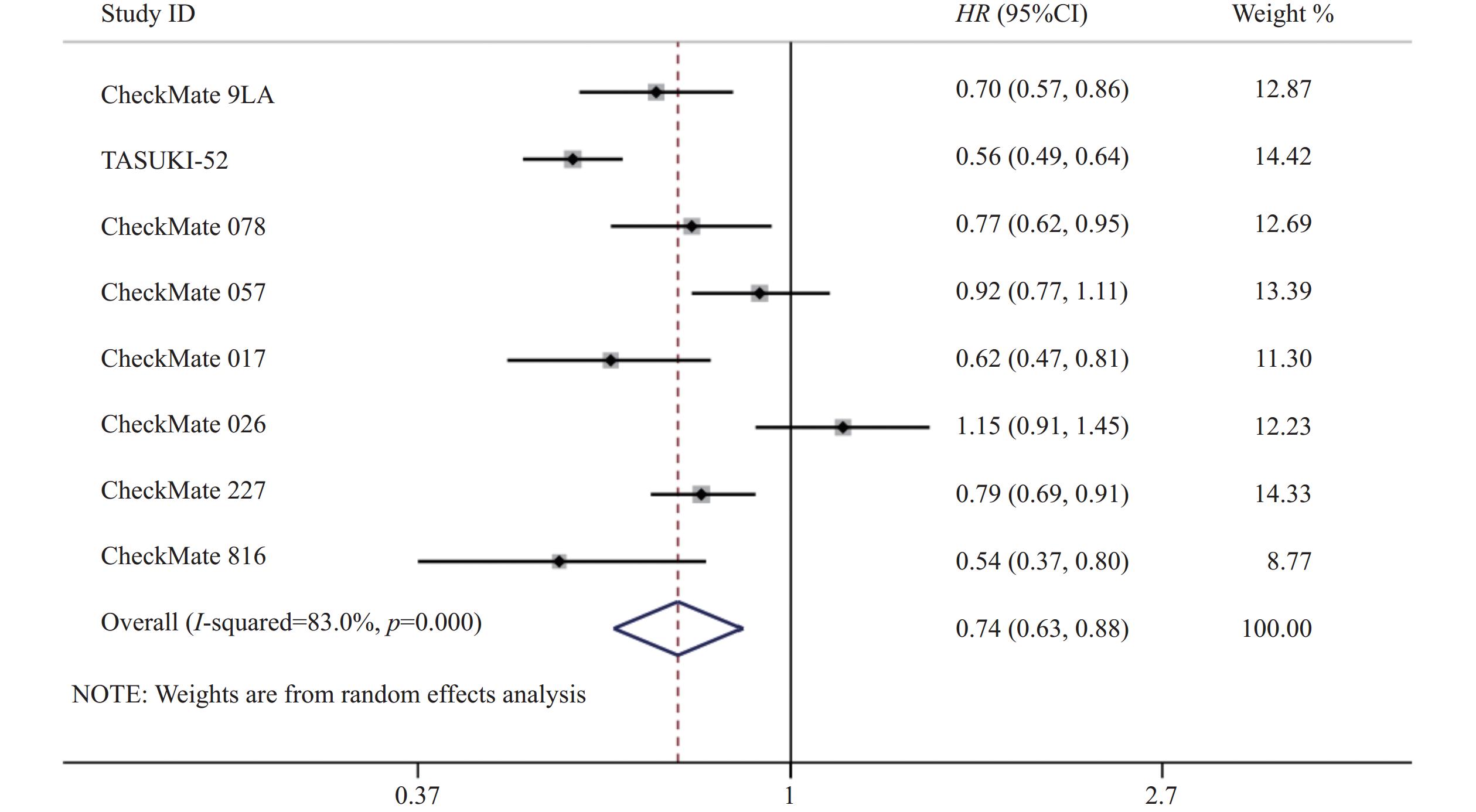
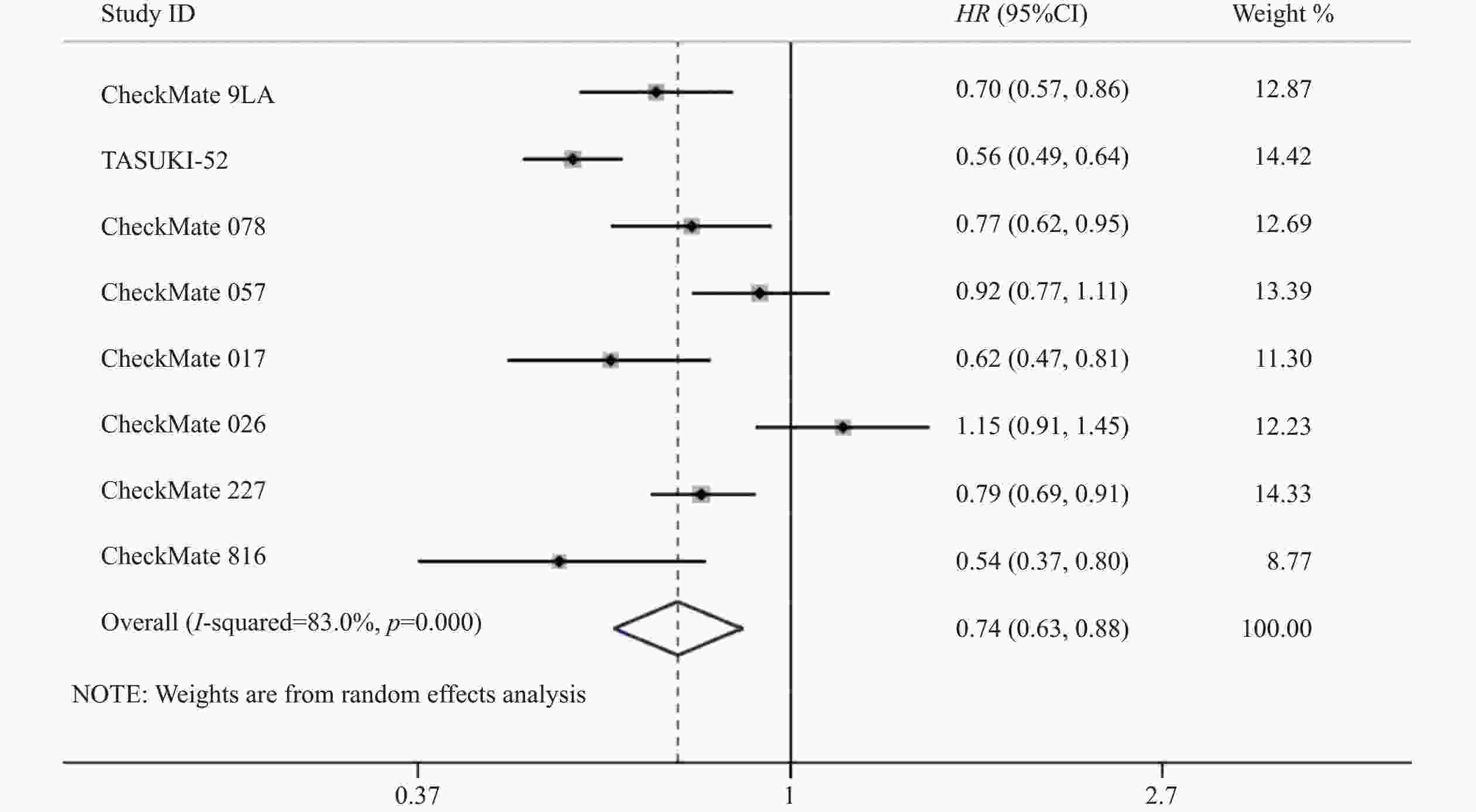
8项研究[7-14]均对PFS进行了统计分析。研究存在统计学异质性(P<0.05,I2=83%),使用随机效应模型,Meta分析结果显示,与对照组相比,试验组患者接受纳武利尤单抗治疗延长了NSCLC患者的PFS,差异有统计学意义(HR=0.74,95%CI=0.63~0.88,P<0.05),因此,使用纳武利尤单抗治疗NSCLC的疗效要优于传统的化疗方案,详见图4。
-
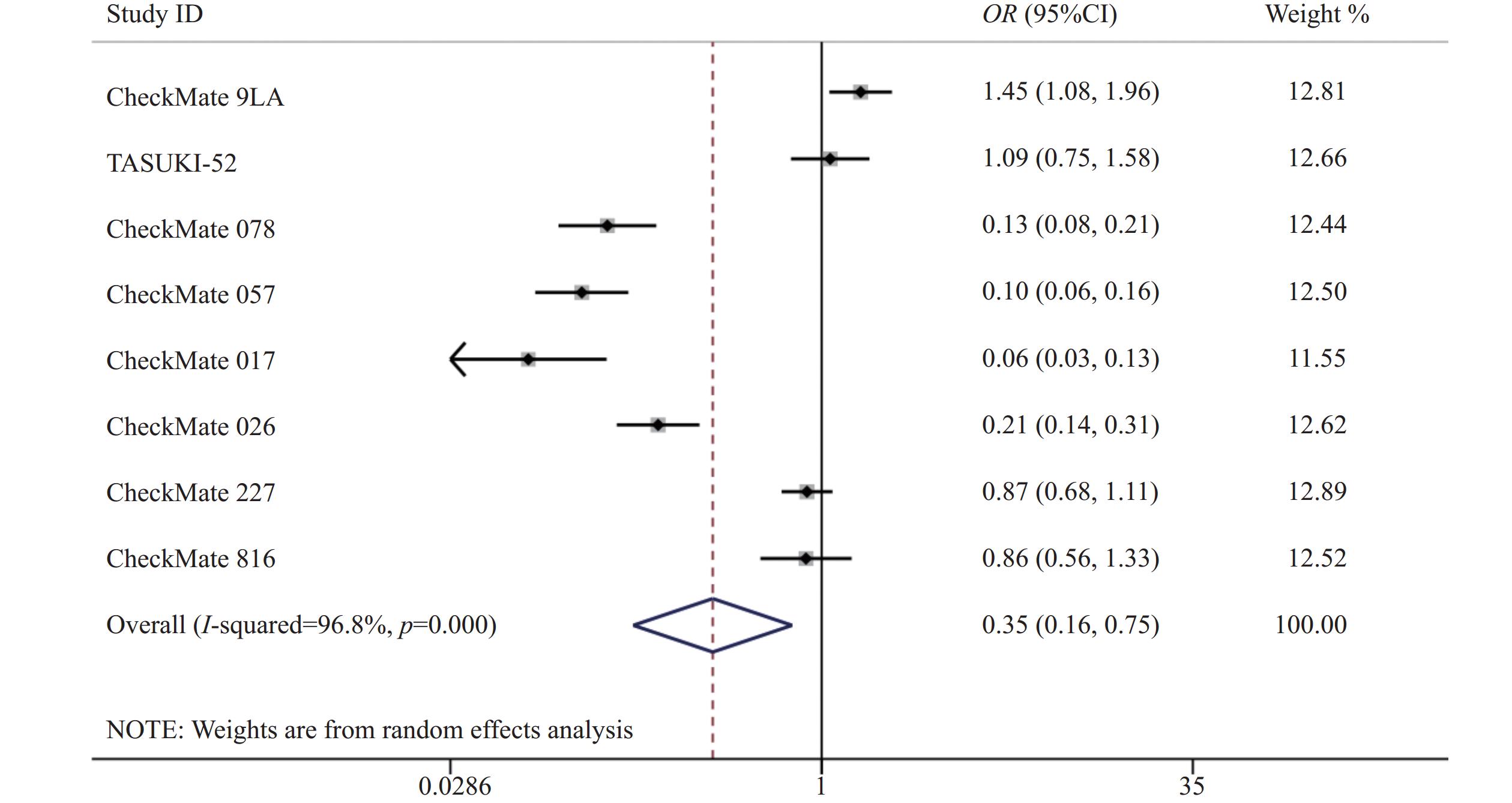
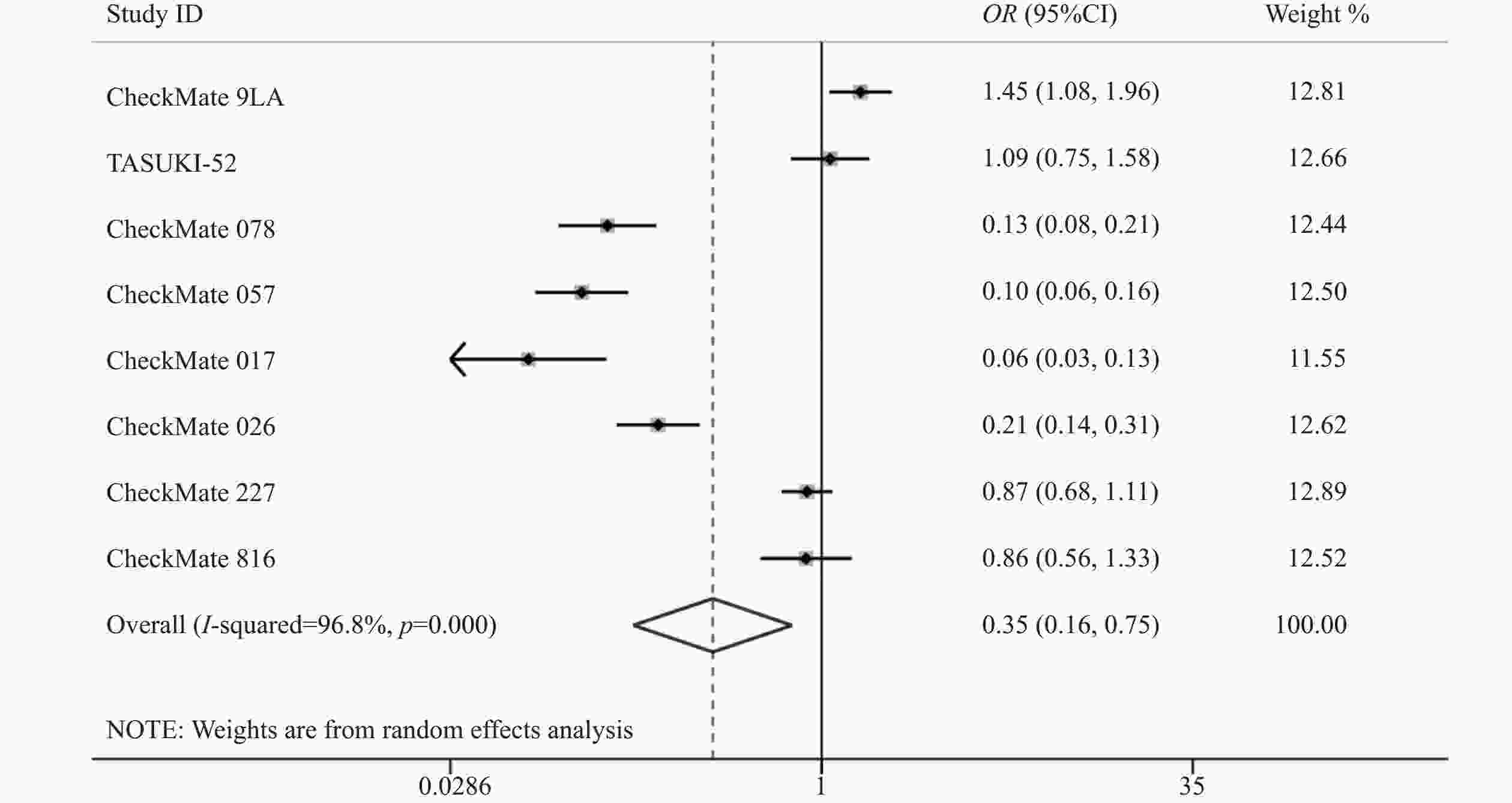
8项研究[7-14]均报道了两组三级以上的不良反应率。各研究间存在统计学异质性(P<0.05,I2=96.8%),使用随机效应模型,Meta分析结果显示,两组患者发生三级以上不良反应差异有统计学意义(OR=0.35,95%CI=0.17~0.75,P<0.05),因此,相对于传统的化疗,使用纳武利尤单抗可能会降低三级以上不良反应发生率,见图5。
-
①7项研究[7-8, 10-14]报道了恶心的发生率,研究间存在异质性(P<0.05,I2=91.7%),使用随机效应模型,两组患者恶心发生率差异有统计学意义(OR=0.38,95%CI=0.22~0.64,P<0.05)。②6项研究[7-8, 10-13]报道了腹泻的发生率,研究间存在异质性(P<0.05,I2=91.9%),使用随机效应模型,两组患者腹泻发生率差异无统计学意义(OR=0.93,95%CI=0.48~1.82,P>0.05)。③8项研究[7-14]报道了中性粒细胞减少的发生率,研究间存在统计学异质性(P<0.05,I2=97.1%),使用随机效应模型,两组患者中性粒细胞减少发生率差异有统计学意义(OR=0.05,95%CI=0.01~0.25,P<0.05)。④7项研究[7-9,11-14]报道了皮疹的发生率,研究间存在统计学异质性(P<0.05,I2=78%),使用随机效应模型,两组患者皮疹的发生率差异有统计学意义(OR=3.85,95%CI=2.05~6.25,P<0.05)。⑤8项研究[7-14]报道了贫血的发生率,研究间存在统计学异质性(P<0.05,I2=96.4%),使用随机效应模型,两组患者皮疹发生率差异有统计学意义(OR=0.16,95%CI=0.06~0.42,P<0.05)。⑥8项研究[7-14]报道了食欲下降的发生率,研究间存在统计学异质性(P<0.05,I2=70.5%),使用随机效应模型,两组患者食欲下降发生率差异有统计学意义(OR=0.57,95%CI=0.42~0.76,P<0.05)。⑦8项研究[7-14]报道了患者疲劳的发生率,研究间存在统计学异质性(P<0.05,I2=86.4%),使用随机效应模型,两组患者疲劳发生率差异有统计学意义(OR=0.54,95%CI=0.35~0.85,P<0.05)。
-
在这项研究中,我们对8项最新的Ⅲ期RCTs研究进行了Meta分析,结果支持纳武利尤单抗单用或联合传统化疗治疗NSCLC的有效性和安全性。更重要的是,Nivolumab相比传统疗法会降低NSCLC患者的三级以上不良反应发生率,降低恶心、中性粒细胞减少、贫血、食欲下降、疲劳的发生率。近年来,越来越多的临床试验报道了PD-1/PD-L1在NSCLC中的疗效和安全性。Zhang 等[15]的Meta分析结果发表于2021年,支持了PD-1/PD-L1在NSCLC中的有效性和安全性。然而,Nivolumab只有2项研究被纳入,其中只有一项研究[12](CheckMate 026试验)提供了完整的已发表数据,而另一项[13](CheckMate 227试验)在进行中。该Meta分析中包含的研究有限,因此,有必要对最新更新的研究数据进行Meta分析。
本研究分析了Nivolumab用于治疗NSCLC的有效性及每项研究发生率较高且共同记录的不良反应的发生率。与以往的研究相比,通过对最新的Ⅲ期RCTs 4 945例患者统计分析,得出的一些创新性结论,对指导临床治疗和后续研究具有新的意义。本研究中所有的文章均为Ⅲ期RCTs并选用平行设计随机对照试验,Cochrane偏倚评估工具2.0版本RoB2.0进行文献质量评价,RoB2.0内容丰富,覆盖全面,为RCTs的证据整合与评价提供了更多的偏倚风险信息。
本研究尚有一定局限性,具体包括:①纳入研究的患者使用纳武利尤单抗并非首次治疗方案,存在基础化疗方案,不同的治疗方案可能对有效性和安全性评价结果存在影响。②该文献检索限定为中文和英文,只检索了已公开发表的文献,且未找到符合要求的国内文献报道。纳入本研究的患者均为国外患者,因此研究结果可能与中国人群存在种族差异。③患者的基线信息如吸烟、组织学亚型、PD-L1表达水平、不同的化疗方案等因素都会影响治疗效果,使评价结果存在偏倚。④本研究中部分结局指标存在异质性,多种因素可能导致异质性,包括临床分期、组织学亚型、PD-L1表达水平、吸烟状况和患者种族,8项临床试验在美洲、亚洲和欧洲不同国家进行,并招募了来自不同种族的患者。为保证研究的同质性,建议后期更新更多临床试验后分析可统一纳入及排除标准。
-
综上所述,相较于传统化疗,纳武利尤单抗治疗NSCLC的疗效和安全性更优,常规单独使用或与化疗联合使用能显著改善患者的总生存期和无进展生存期,且不会增加传统化疗引起的不良反应(如恶心、中性粒细胞减少、贫血、食欲下降、疲劳等)。然而,纳武利尤单抗增加了免疫相关不良反应的风险如皮疹的发生,应引起关注,临床医生应仔细平衡纳武利单抗的疗效、毒性和成本,以优化临床结果。
Efficacy and safety of nivolumab in the treatment of non-small cell lung cancer:a meta-analysis
doi: 10.12206/j.issn.2097-2024.202310044
- Received Date: 2023-10-23
- Rev Recd Date: 2024-03-12
- Available Online: 2024-10-24
- Publish Date: 2024-10-25
-
Key words:
- nivolumab /
- non-small cell lung cancer /
- Meta-analysis /
- efficacy /
- safety
Abstract:
| Citation: | LIU Liyan, YU Xiaocui, SUN Chuanduo. Efficacy and safety of nivolumab in the treatment of non-small cell lung cancer:a meta-analysis[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(10): 451-456. doi: 10.12206/j.issn.2097-2024.202310044 |







 DownLoad:
DownLoad: