-
阿霉素(DOX)是一种蒽环类化疗抗生素,尽管其能有效治疗多种实体肿瘤和血液恶性肿瘤,但对肿瘤细胞和正常细胞的无差别攻击导致的毒副作用,使其临床应用受到限制[1-3]。特别是剂量依赖性心脏毒性最为突出,研究认为氧化应激是诱导心脏毒性的重要机制。其他常见的毒副作用还包括急性恶心呕吐、口腔炎、胃肠道紊乱、脱发、神经障碍和骨髓发育不全等[4-6]。同时,多药耐药的出现是DOX使用的附加问题[2]。因此,需要寻求DOX新的剂型结构来降低药物毒副作用带来的风险。
金纳米粒(AuNPs)具有毒副作用小、生物相容性良好的特性,且其具有粒径大小可调控、表面易修饰、高效的光热转化等优势,常被用于肿瘤的诊断和治疗[7-8]。AuNPs表面可修饰连接抗肿瘤药物、靶向肽、荧光物质或功能化基团等,实现精准治疗和实时监测[9]。常用的修饰配体为巯基(-SH),以形成Au-S键的方式连接在AuNPs表面,也可利用氨基与AuNPs的静电作用构建载体,但有研究表明,巯基比氨基作用更强,不易断裂[10]。
本研究制备了一种载DOX的mPEG修饰金纳米粒AuNPs-mPEG@DOX,验证了该纳米载体在保持对乳腺肿瘤细胞杀伤活性的同时,降低了DOX对正常乳腺细胞的毒副作用,为后续AuNPs连接DOX用于肿瘤治疗提供参考。
-
ZD-85A型气浴恒温震荡培养箱(上海启前电子科技有限公司);十万分之一电子天平(瑞士METTLER TOLEDO公司);超纯水系统、数显pH计(均为德国Sartorius公司);HDC-15K型离心机(上海泰坦科技股份有限公司);Zetasizer nano ZS型激光粒度分析仪(英国Malvern公司);1260 Infinity II型高效液相色谱仪(安捷伦科技有限公司);UV1800型紫外可见分光光度仪(中国TECHCOMP公司)。
-
金纳米粒(20 nm,南京东纳生物科技公司);HS-mPEG5K(上海芃硕生物科技有限公司);HS-DOX(西安瑞禧生物科技有限公司);乙腈、三乙胺、磷酸,均为色谱纯(国药集团化学试剂有限公司)。
-
人乳腺细胞(MCF-10A)、人乳腺癌细胞(MCF-7),均由海军军医大学药学系药剂学教研室提供。
-
用纯水溶解HS-mPEG5K得到浓度为1 mg/ml的HS-mPEG5K溶液,向1 ml AuNPs(50 μg/ml)中加入5 μl HS-mPEG5K溶液,4 ℃孵育8 h,孵育结束后于4 ℃、以12000 g离心10 min,弃去含未反应HS-mPEG5K的上清液,纯水重悬。
-
用纯水溶解HS-DOX得到浓度为44.7 μg/ml的HS-DOX溶液,取上步1 ml AuNPs-mPEG重悬液离心,弃去上清液,用0.75 ml纯水重悬后,加入0.25 ml HS-DOX溶液, 4 ℃孵育8 h,离心弃去含未反应HS-DOX的上清液,纯水重悬。
-
取1 ml AuNPs、AuNPs-mPEG和AuNPs-mPEG@DOX溶液于马尔文Zeta电位样品池,使用激光粒度分析仪测定粒径和Zeta电位,每个样品测量3次。
-
取1 ml AuNPs、AuNPs-mPEG和AuNPs-mPEG@DOX溶液于石英皿中,扫描波长400 nm~700 nm,以纯水作为各样品的纯溶剂参比进行光谱分析。
-
向1 ml 50 μg/ml AuNPs中加入5 μl HS-mPEG5K溶液,4 ℃孵育8 h后离心去上清,分别用0.9375、0.875、0.75、0.5 ml 纯水重悬AuNPs-mPEG,再分别加入0.0625、0.125、0.25、0.5 ml HS-DOX溶液,得到投药浓度分别为2.79、5.59、11.18、22.35 μg/ml的反应体系,4 ℃孵育8 h,离心取上清液,通过高效液相色谱法(HPLC)对上清液进行定量检测,考察HS-DOX投药浓度对AuNPs-mPEG@DOX吸附率和载药量的影响。具体检测方法见“2.3”项。
-
色谱柱:Extend-C18柱(4.6 mm×150 mm,5 µm);流动相:水(0.1%三乙胺,磷酸调节pH 3.0)∶乙腈=75∶25;流速:1.0 ml/min;进样量:10 µl;柱温:室温;检测波长:254 nm。
-
对照品溶液:精密称取HS-DOX 4.47 mg,加纯水在100 ml量瓶中定容,0.22 μm微孔滤膜过滤,取续滤液,得到浓度为44.7 μg/ml的HS-DOX对照品溶液。
供试品溶液:1 ml AuNPs-mPEG离心沉淀用0.75 ml纯水重悬,加入0.25 ml HS-DOX(44.7 μg/ml),4 ℃孵育8 h,离心取上清液,0.22 μm微孔滤膜过滤,取续滤液作为供试品溶液。
-
配制以下溶液:A:5.59 μg/ml HS-DOX对照品溶液;B:1 ml AuNPs-mPEG离心沉淀用0.75 ml纯水重悬,加入0.25 ml纯水混合,4 ℃孵育8 h,离心取上清液,0.22 μm微孔滤膜过滤,为空白基质溶液;C:供试品溶液。
-
用纯水稀释HS-DOX对照品溶液,制得HS-DOX浓度为1.40、2.79、5.59、11.18、22.35 μg/ml的标准工作液。以峰面积(A)为纵坐标,质量浓度(μg/ml)为横坐标进行线性回归。
-
分别选取低、中、高3个质量浓度(1.40、5.59、22.35 μg/ml)的HS-DOX标准工作液,1 d内进样3次,每次每个浓度连续进样5次,计算峰面积的相对标准偏差(RSD),考察日内精密度;连续进样3 d,每次每个浓度连续进样5次,考察日间精密度。
-
取对照品溶液和供试品溶液于室温下储存,分别于0、4、8、12、16、24 h测定HS-DOX的含量,计算RSD。
-
取9份供试品各1 ml,分别加入1 ml的含8.12、10.15、12.18 μg的HS-DOX对照品溶液混合,每组平行制备3份,得到80%、100%、120%加样量的加样供试品溶液。根据以下公式计算HS-DOX的加样回收率。
-
测定离心后的上清液,根据以下公式计算吸附率和载药量。
-
分别取MCF-10A和MCF-7细胞,用DMEM混合培养基调整细胞密度为5×104 cells/ml,以每孔0.1 ml的体积铺板于96孔板,37 ℃下孵育24 h。分别用相同摩尔浓度的AuNPs-mPEG@DOX和游离DOX(0.02、0.06、0.18、0.53、1.58、4.75、14.25 μmol/L)处理24 h和48 h,每种药物浓度重复3个孔。用10%的CCK8溶液染色30 min。
-
实验结果用(
$\bar x $ ±s)表示。采用GraphPad Prism v9 (San Diego, USA)软件进行统计分析。两组间比较采用Two-Way ANOVA ,P≤0.05认为差异有统计学意义。 -
AuNPs、AuNPs-mPEG和AuNPs-mPEG@DOX的粒径分布和Zeta电位如表1所示,3种纳米颗粒的粒径逐渐增大,Zeta电位由负电荷转为正电荷,最终得到水动力直径为(46.12±0.49) nm,电位为(18.60±1.51) mV的AuNPs-mPEG@DOX。AuNPs与HS-mPEG5K孵育后,部分柠檬酸根被甲氧基聚乙二醇取代,连接了PEG长链的AuNPs-mPEG更稳定不易聚集,粒径增大,负电荷减少;连接HS-DOX后,由于阿霉素含有氨基带正电,因而制备得到带正电荷的纳米颗粒。
纳米颗粒 平均粒径(nm) 多分散系数 Zeta电位(mV) AuNPs 28.31±0.37 0.38±0.02 −25.30±0.99 AuNPs-mPEG 43.32±1.40 0.38±0.07 −22.20±0.49 AuNPs-mPEG@DOX 46.12±0.49 0.38±0.04 18.60±1.51 -
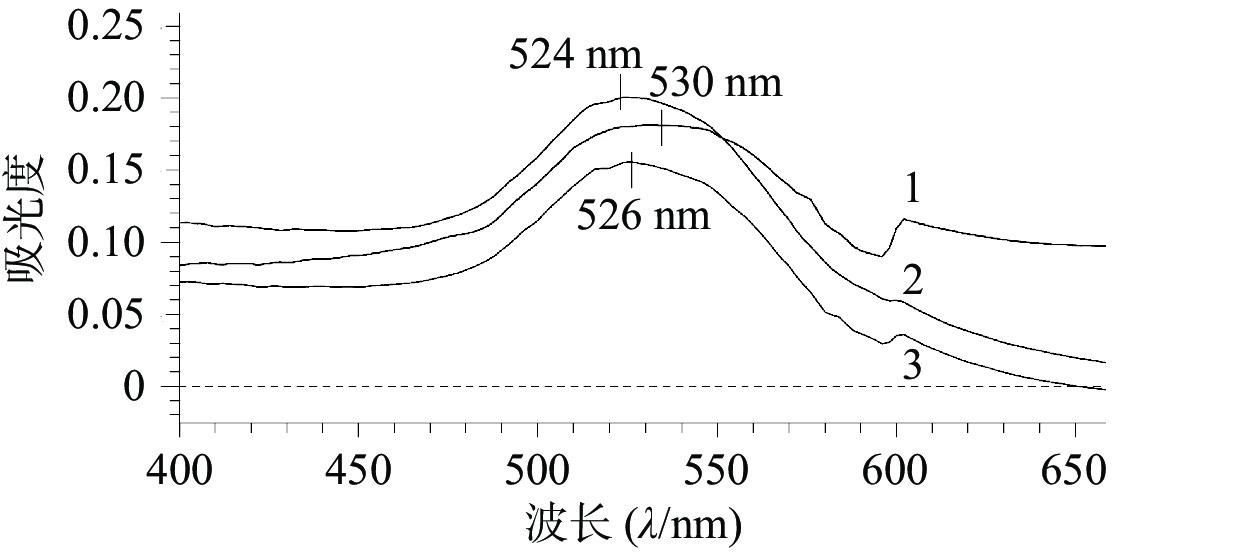
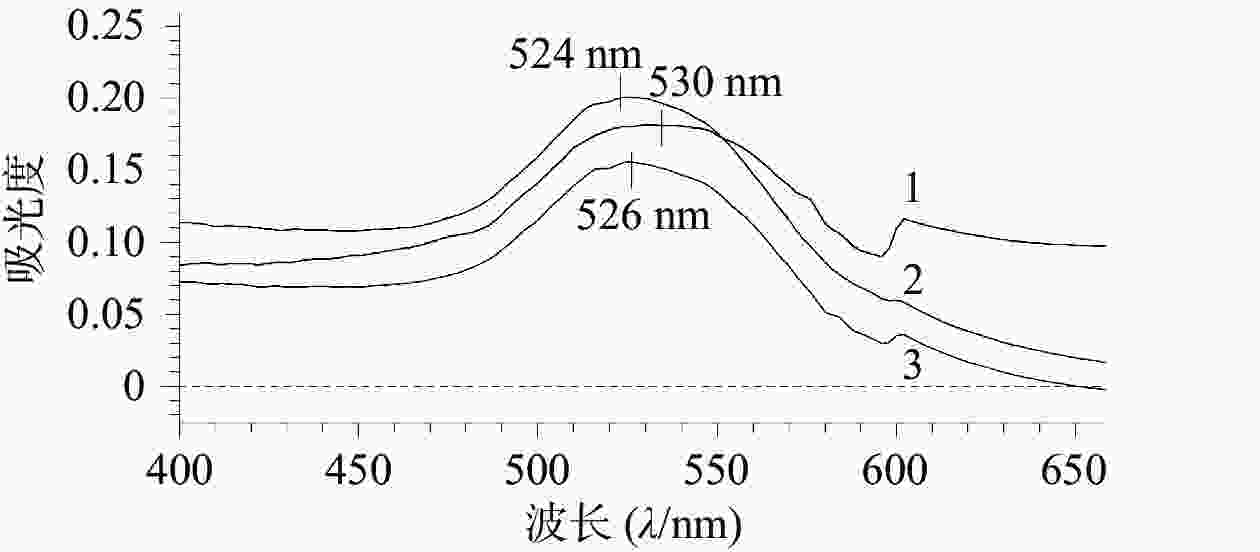
如图1所示,AuNPs、AuNPs-mPEG和AuNPs-mPEG@DOX的最大吸收波长分别为524、526、530 nm,随着HS-mPEG5K和HS-DOX被修饰到AuNPs上,最大吸收波长依次出现红移,表明mPEG5K和DOX通过HS键成功连接在AuNPs上。
-
为提高纳米载体的吸附率和载药量,考察了HS-DOX投药浓度对AuNPs-mPEG@DOX吸附率和载药量的影响。如表2所示,随着HS-DOX的投药浓度增加,载体的吸附率和载药量都逐渐升高,当投药浓度为11.18 μg/ml时,纳米载体的吸附率和载药量达到最大值,继续提高HS-DOX的投药浓度,载药量不再上升,吸附率降低。表明HS-DOX投药浓度在11.18 μg/ml时,AuNPs表面吸附可能达到饱和,因而选择11.18 μg/ml投药浓度用于制备AuNPs-mPEG@DOX。
HS-DOX投药浓度
(μg/ml)吸附浓度
(μg/ml)吸附率
(%)载药量
(%)2.79 0.11±0.02 3.96±0.57 0.22±0.03 5.59 0.51±0.16 9.20±2.89 1.02±0.32 11.18 1.03±0.32 9.21±2.88 2.01±0.62 22.35 0.92±0.02 4.13±0.09 1.81±0.04 -
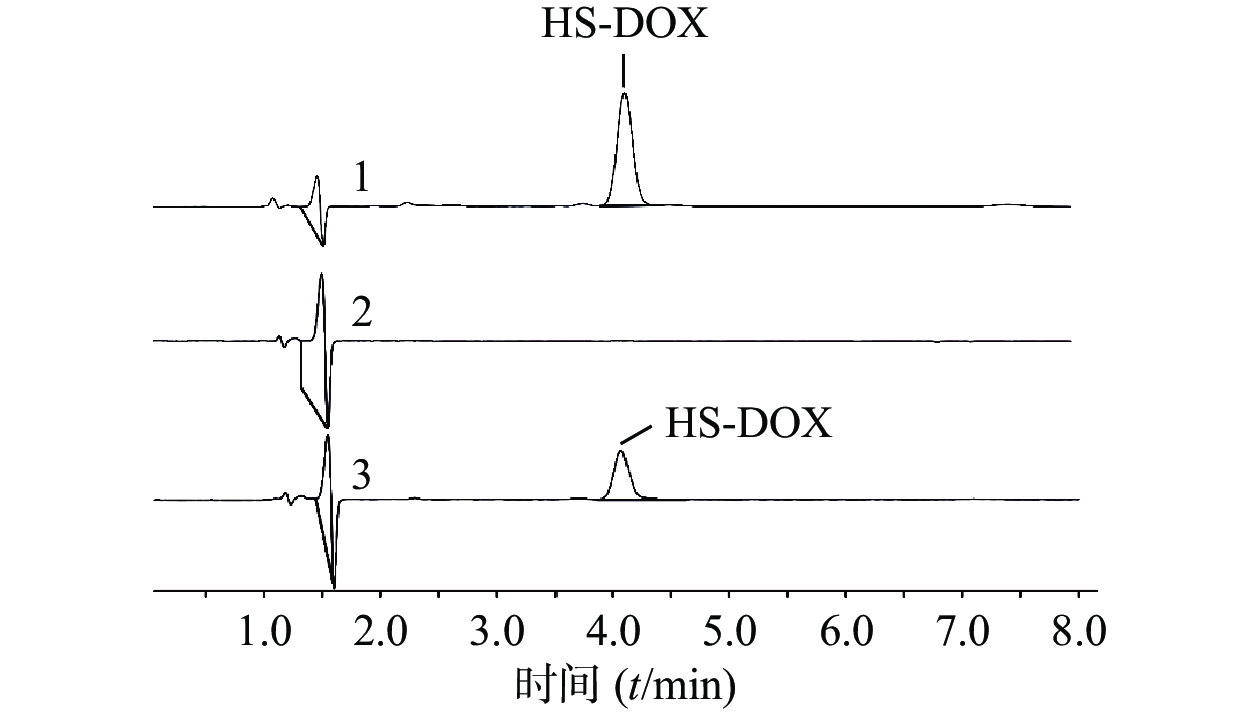
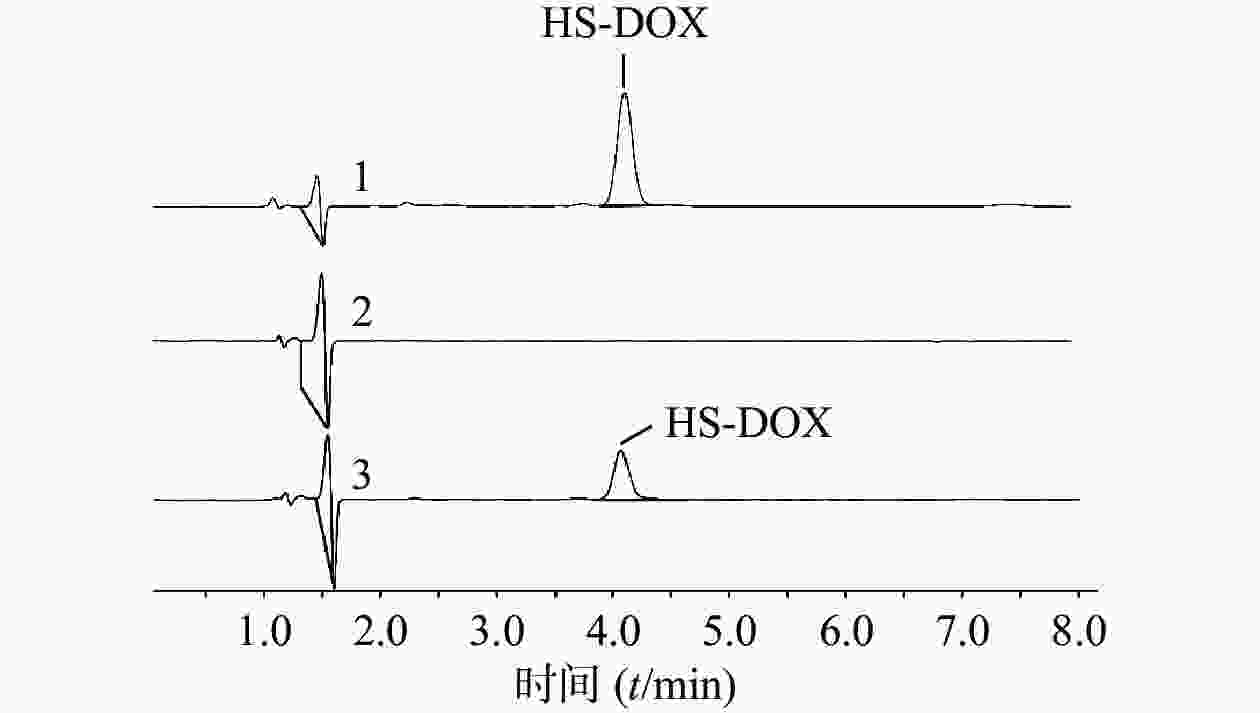
首先考察方法专属性,实验表明,制备过程未对HS-DOX的测定产生影响(图2)。进一步考察HS-DOX的线性,得线性回归方程为y=17.884 x−5.3651,r=0.9999,证明该液相色谱方法在1.40~22.35 μg/ml浓度范围内线性良好。分别考察了1.40、5.59、22.35 μg/ml 3个低、中、高浓度的精密度,见表3,日内精密度和日间精密度均<2.0%,符合精密度要求。对照品溶液和供试品溶液在24 h内基本稳定,其RSD分别为0.78%和1.01%。HS-DOX平均加样回收率为99.06%,RSD为0.83%,回收率符合要求(表4)。结果表明,该液相方法准确可靠,可用于HS-DOX的定量检测。
浓度
(μg/ml)日内精密度 日间精密度 $\bar x $±s RSD(%) $\bar x $±s RSD(%) 1.40 1.41±0.02 1.70 1.41±0.03 1.79 5.59 5.52±0.08 1.40 5.55±0.10 1.83 22.35 22.37±0.19 0.84 22.36±0.17 0.77 加入量(m/μg) 测得量(m/μg) 原有量(m/μg) 回收率(%) 8.12 18.13 10.03 99.75 18.11 10.10 98.65 17.97 9.98 98.40 10.15 20.42 10.21 100.59 20.33 10.30 98.82 20.00 10.02 98.33 12.18 21.95 9.95 98.52 22.18 10.20 98.36 22.19 9.99 100.16 平均回收率(%) 99.06 RSD(%) 0.83 -
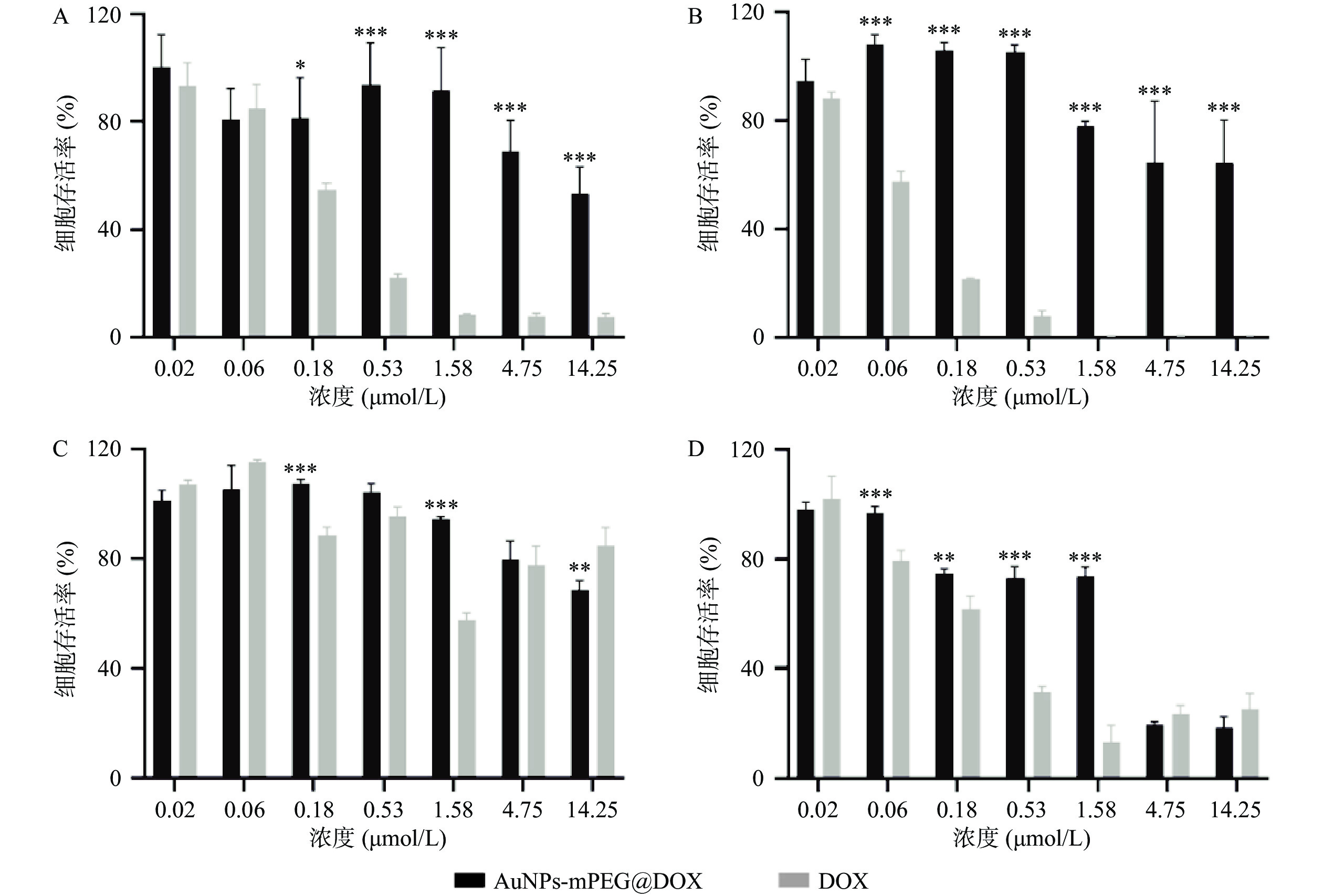
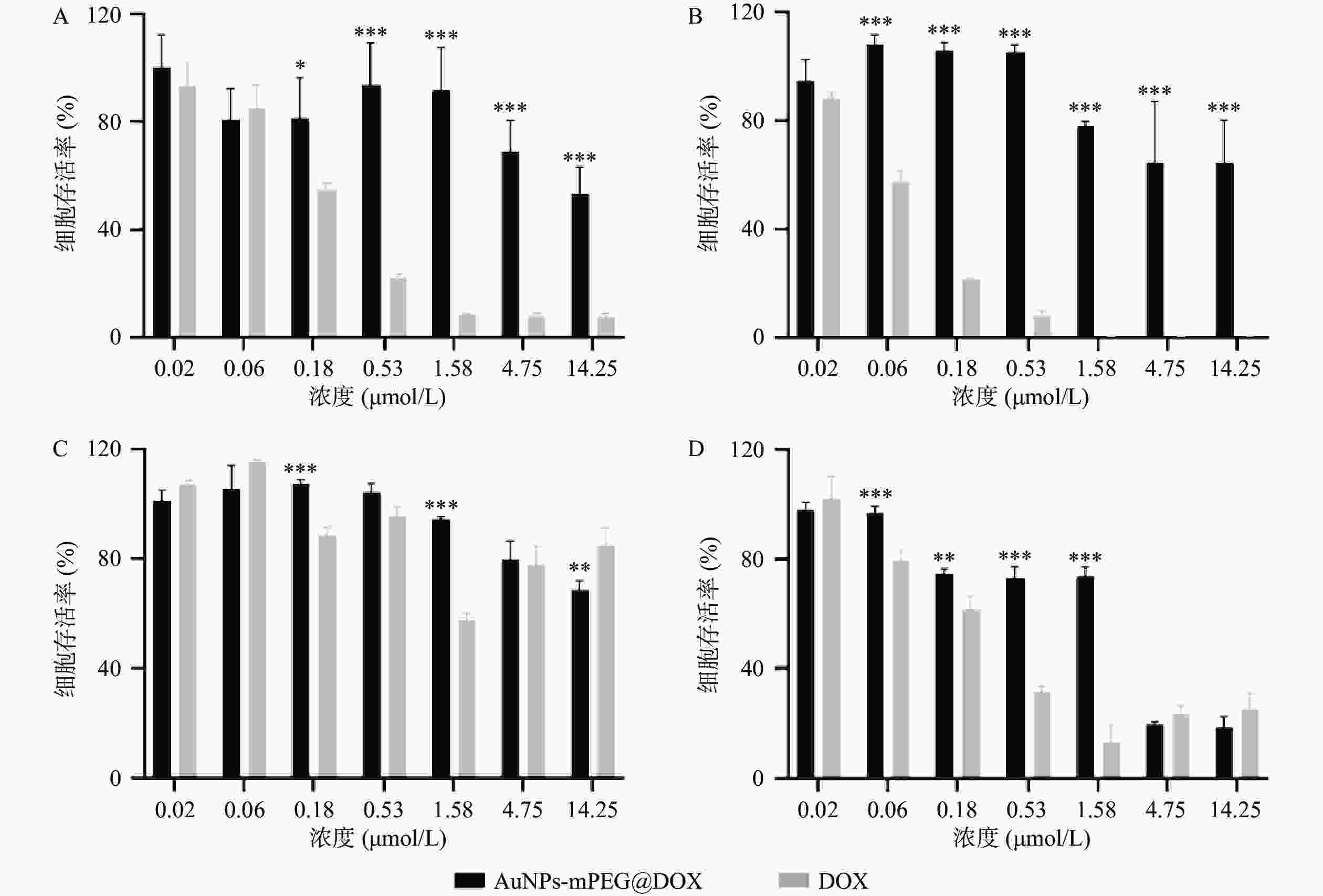
AuNPs-mPEG@DOX和DOX对MCF-10A和MCF-7细胞的24 h和48 h毒性作用如图3所示,随着DOX浓度的增加以及药物作用时间延长,MCF-10A和MCF-7细胞的存活率皆降低。在相同DOX浓度下,AuNPs-mPEG@DOX比游离DOX对正常乳腺细胞MCF-10A的毒性作用明显降低,可起到减小DOX毒副作用的目的。当DOX浓度<4.75 μmol/L作用于乳腺癌细胞MCF-7时,游离DOX表现出比AuNPs-mPEG@DOX明显的细胞毒性作用;在DOX浓度≥4.75 μmol/L的情况下,AuNPs-mPEG@DOX与游离DOX表现出无差异的细胞毒性作用。结果表明将DOX修饰于AuNPs上,由于Au-S键稳定存在,以及PEG的长链保护作用,DOX与细胞的直接接触面积减小,可降低对正常细胞的毒副作用。由于肿瘤细胞的高间质液压大,尺寸较大的AuNPs-mPEG@DOX较游离DOX不易被泵回血液和细胞胞吐,在高浓度时,与DOX肿瘤细胞杀伤作用相当。
-
DOX在杀伤肿瘤细胞的同时也会损害机体的正常细胞,其随剂量增加会对心脏、大脑、肝脏和肾脏等器官产生不良反应。同时,它还会损害免疫系统,增加病人对病原体的易感性,削弱他们的愈合能力。本文制备了载DOX的mPEG修饰金纳米粒AuNPs-mPEG@DOX,粒径为(46.12±0.49) nm,电位为(18.60±1.51) mV,最大吸收波长为530 nm。成功建立了可用于检测AuNPs-mPEG@DOX未吸附HS-DOX含量的HPLC方法,在HS-DOX投药浓度为11.18 μg/ml时有最大吸附率和载药量。该纳米载体能明显降低DOX对正常乳腺细胞的毒副作用,且当浓度达到一定值时,具有与游离DOX相同的肿瘤细胞杀伤作用,为后续开发AuNPs连接DOX的肿瘤治疗方案奠定了良好的基础。
纳米颗粒被认为是选择性增加肿瘤细胞内药物积累从而减少毒副作用的重要途径。AuNPs作为无机纳米颗粒的一种,因其独特性质被广泛应用于药物递送、肿瘤诊断和治疗领域。除AuNPs本身结构可降低DOX毒副作用,也可通过在AuNPs表面修饰核酸适配体[11-12]、肿瘤穿透肽[13]等有效靶向和进入肿瘤细胞,减少DOX对正常细胞的损害;或利用肿瘤部位的特殊环境,通过响应型功能键将DOX连接于AuNPs上,如低pH[14]、过表达酶[15]和高水平的GSH[16]等来调控载体尺寸大小和DOX释放,提高载药纳米颗粒在肿瘤部位蓄积量,继而提高抗肿瘤作用。此外,也可以通过将DOX和Varlitinib与PEGAuNPs结合[17],来增强DOX对肿瘤细胞的抑制作用,降低对正常细胞的毒性。虽然利用AuNPs载DOX同时降低其毒性的策略目前已被广泛研究,但其粒径大小、表面电荷和各类形状等因素对生物分布和细胞摄取的影响,AuNPs表面有限吸附能力带来的载体量过大,以及进入细胞后的代谢途径等问题还需要进行更多的探索。
Preparation and cytotoxicity of doxorubicin-containing gold nanoparticles
doi: 10.12206/j.issn.2097-2024.202308043
- Received Date: 2023-08-21
- Rev Recd Date: 2023-10-19
- Available Online: 2024-02-28
- Publish Date: 2024-02-25
-
Key words:
- gold nanoparticles /
- doxorubicin /
- breast tumor /
- toxicity
Abstract:
| Citation: | XU Ziyi, SUN Yuhan, FAN Li, LU Guangzhao, ZHANG Yingnan, ZHANG He. Preparation and cytotoxicity of doxorubicin-containing gold nanoparticles[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(2): 73-77, 81. doi: 10.12206/j.issn.2097-2024.202308043 |








 DownLoad:
DownLoad: