-
光动力治疗(PDT)基于光辐照聚集光敏剂的肿瘤组织,由光敏剂诱发光动力反应形成单线态氧(1O2)等活性氧(ROS),通过对肿瘤细胞和肿瘤血管的直接杀伤及激活机体系统免疫反应等多种机制发挥抗肿瘤作用[1-3]。二氢卟吩及菌绿素类光敏剂是PDT新药研究的热点[4-8]。其中,已获批上市的代表药物有他拉泊芬(talaporfin)和帕利泊芬(padeliporfin)等[9, 10]。
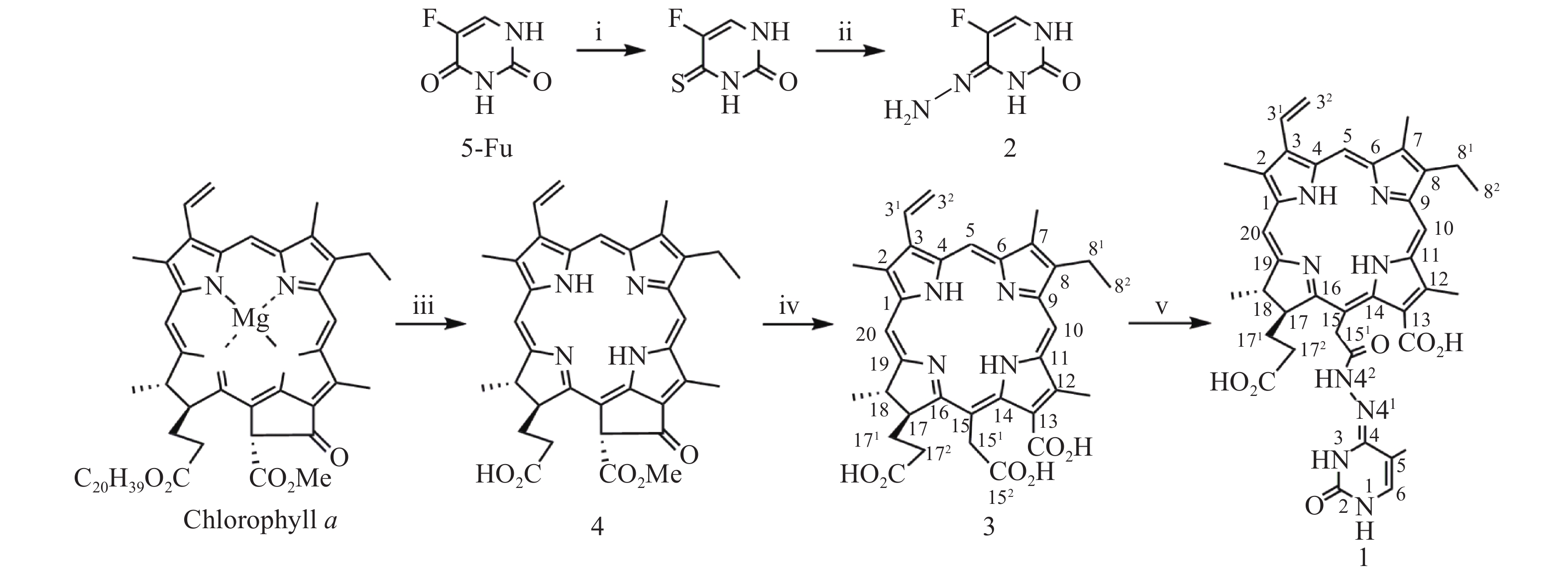
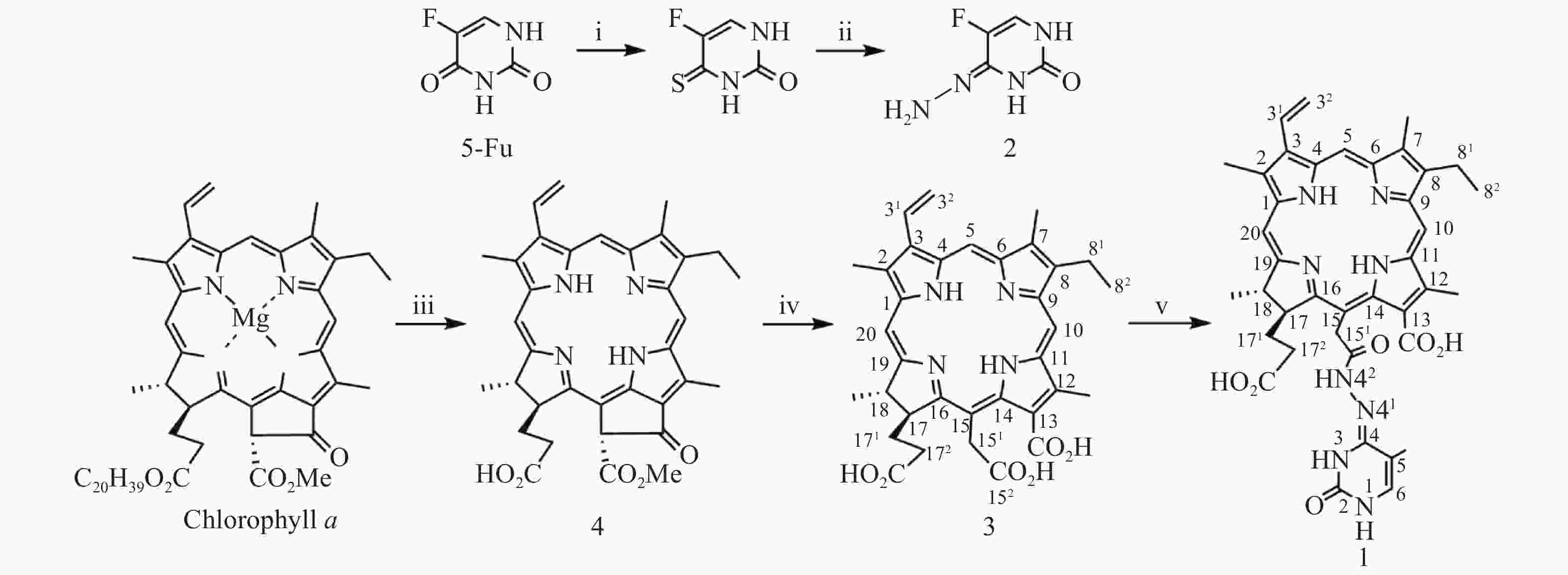
光敏剂作为结构非特异性药物,存在缺乏肿瘤靶向性摄入和明确的作用药靶等缺陷。此外,PDT受制于局部治疗,对浸润较深的肿瘤组织,及已发生转移的肿瘤疗效有限。目前,PDT和化疗联用是克服上述缺陷,提高PDT疗效最为普遍和有效的策略之一。研究表明,抗代谢化疗药物氟尿嘧啶(5-Fu)与PDT联用具有协同抗肿瘤作用[11-13]。据此,我们设想利用在肿瘤微环境下能响应性断裂的连接基团(linker)将光敏剂与化疗药物偶联,希望实现二者在肿瘤组织的靶向释放,从而发挥其PDT和化疗协同抗肿瘤作用。酰腙键是酸敏感化学键,常被用来连接载体,以药物制备智能药物载体。这种药物载体到达肿瘤细胞的内涵体或溶酶体中时,会发生酸性水解将药物有效释放出来。因此,本文针对肿瘤微环境呈弱酸性的特点,采用药物化学最经典的前药设计策略,以脱镁叶绿素a(Phephorbide a)粗提物经酸碱降解制得的二氢卟吩e6(3)[14]为先导光敏剂,通过其152-羧基与抗肿瘤药物5-Fu以酸敏感酰腙键连接,设计合成pH响应型光化疗协同抗肿瘤光敏剂二氢卟吩e6-偕氟尿嘧啶(1),并考察其体外PDT抗肿瘤活性和pH响应性5-Fu释放,及其对黑色素瘤B16-F10和肝癌HepG2细胞的光动力抗癌活性及其作用机制,以期获得高效、低毒的PDT治癌药物候选药物,合成路线见图1。
-
用Bruker MSL-600型核磁共振仪测定1H NMR,CD3OD为溶剂;用API-3000 LC-MS型电喷雾质谱仪测定质谱(ESI-MS);用岛津UV-160型紫外分光光度计测定UV吸收谱;用日立F-7000荧光分光光度计测定荧光发射谱;用Shimazu LC-20AD HPLC仪测定化合物1的相对纯度及其5-Fu的体外释放。色谱柱型号为Waters Xterra C18柱,流动相:乙腈-0.3%乙酸水溶液(80 : 20);流速:1.0 ml/min;检测波长:400 nm(化合物1的相对纯度)或254 nm(5-Fu释放);柱温:30 ℃;进样量:20 μl。柱色谱分离用TELEDYNE ISCO的快速制备色谱Combi Flash@Rf+仪,硅胶H作为固定相。PDT抗癌活性测试使用BWT半导体激光仪(北京凯普林,波长为660 nm);用流式细胞仪(BD Accuri C6,美国)(激发波长:488 nm,发射波长:525 nm)检测受试肿瘤细胞样品的ROS水平、细胞凋亡率和细胞周期阻滞。
二氢卟吩e6(3)按照文献[14]的方法制备;其它实验用材料和化学试剂均为市售商品。
-
取氟尿嘧啶(0.2 g,1.563 mmol)溶于无水吡啶(10 ml),加入五硫化二磷(0.298 g,1.563 mmol),加热回流12 h。反应完毕,减压回收溶剂,残物加乙酸乙酯溶解(100 ml),用0.1 mol/L HCl洗涤(50 ml×2),无水Na2SO4干燥,减压除去溶剂得4-硫代-5-氟尿嘧啶粗品。上述4-硫代-5-氟尿嘧啶粗品加甲醇(10 ml)溶解,于0 ℃下滴加N2H4·H2O(0.316 g,6.252 mmol),室温继续搅拌2 h。反应完毕,减压抽滤,P2O5真空干燥得固体化合物5-氟尿嘧啶-4-腙(2)中间体,直接用于下步反应。取二氢卟吩e6(0.1 g,0.168 mmol)溶于无水DMF(10 ml),加1-乙基-(3-二甲氨基丙基)碳二亚胺盐酸盐(EDC·HCl)(0.035 g,0.183 mmol),室温搅拌反应6 h后再加入中间体2(0.031 g,0.218 mmol),继续搅拌36 h。反应完毕,反应液加入10倍体积量乙酸乙酯,饱和NaCl水溶液洗涤(50 ml×3),无水Na2SO4干燥,减压回收溶剂所得固体经快速制备色谱梯度洗脱分离纯化(流动相为二氯甲烷/甲醇/甲酸=15∶1∶0.1~8∶1∶0.1)得黑色固体1纯品0.048 g,产率39.6%。UV-vis λmax (MeOH, nm) (ε, M−1cm−1):660 (3.15×104), 510 (0.82×104), 402 (8.13×104)。1H-NMR (600 MHz, CD3OD, δ, ppm): 9.79 (s, 1H, 10-CH), 9.73 (s, 1H, 5-CH), 9.07 (s, 1H, 20-CH), 8.19 (dd, J = 18.0, 12.0 Hz, 1H, 31-CH), 7.29 (s, 1H, 5-Fu的6-CH), 6.38 (d, J = 18.0 Hz, 1H, 32-CHB), 6.15 (d, J = 12.0 Hz, 1H, 32-CHA), 5.35 (s, 2H, 151-CH2), 4.65 (m, 2H, 17-CH和18-CH), 3.84 (q, J = 7.5 Hz, 2H, 81-CH2), 3.63 (s, 3H, 12-CH3), 3.53 (s, 3H, 2-CH3), 3.30 (s, 3H, 7-CH3), 2.3~2.0 (m,4H , 171-CH2 和172-CH2), 1.76 (m, 6H¸ 18-CH3和82-CH3)。MS (ESI+) m/z: 723.63 (M+H)+ (100%)。元素分析(C38H39N8O6F,%)计算值:C 63.16, H 5.40, N 15.48;实测值:C 63.34, H 5.38, N 15.43。HPLC测定纯度:95.2%。
-
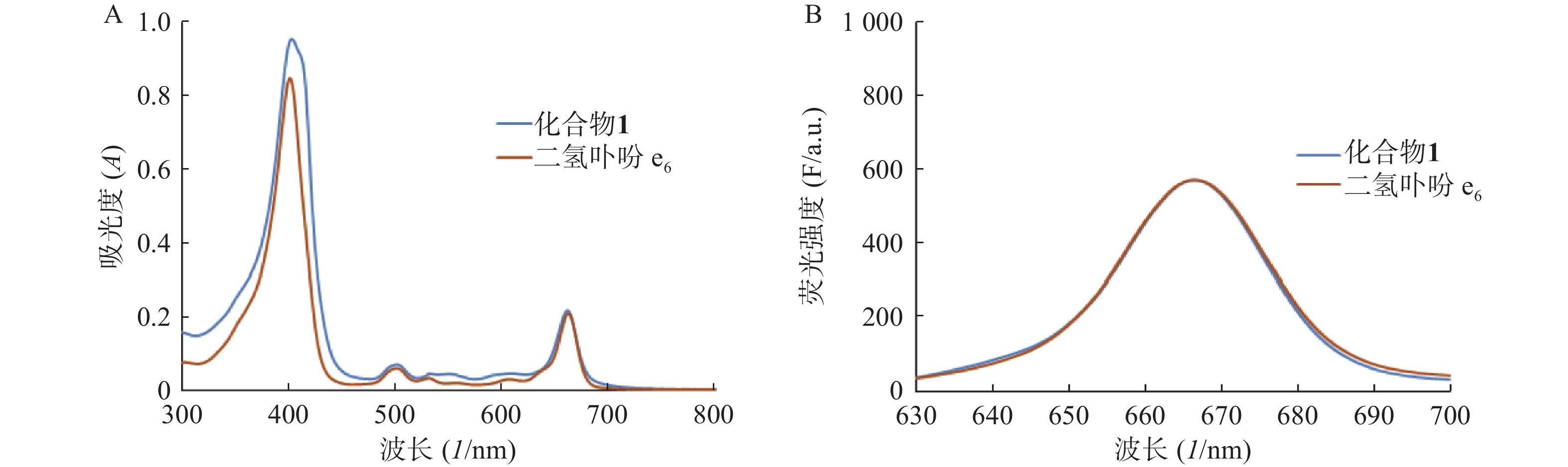
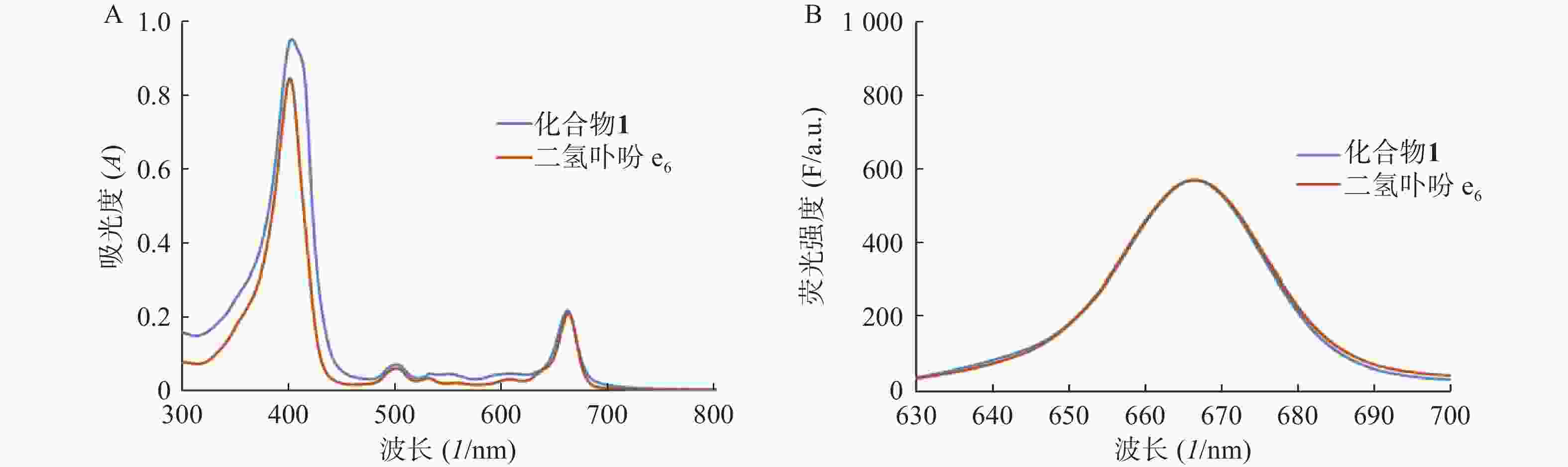
分别测定目标化合物1及其先导化合物二氢卟吩e6(3)的甲醇溶液(10 μmol/L)在300~800 nm处的紫外吸收谱和激发波长为400 nm的荧光发射光谱,结果见图2。
-
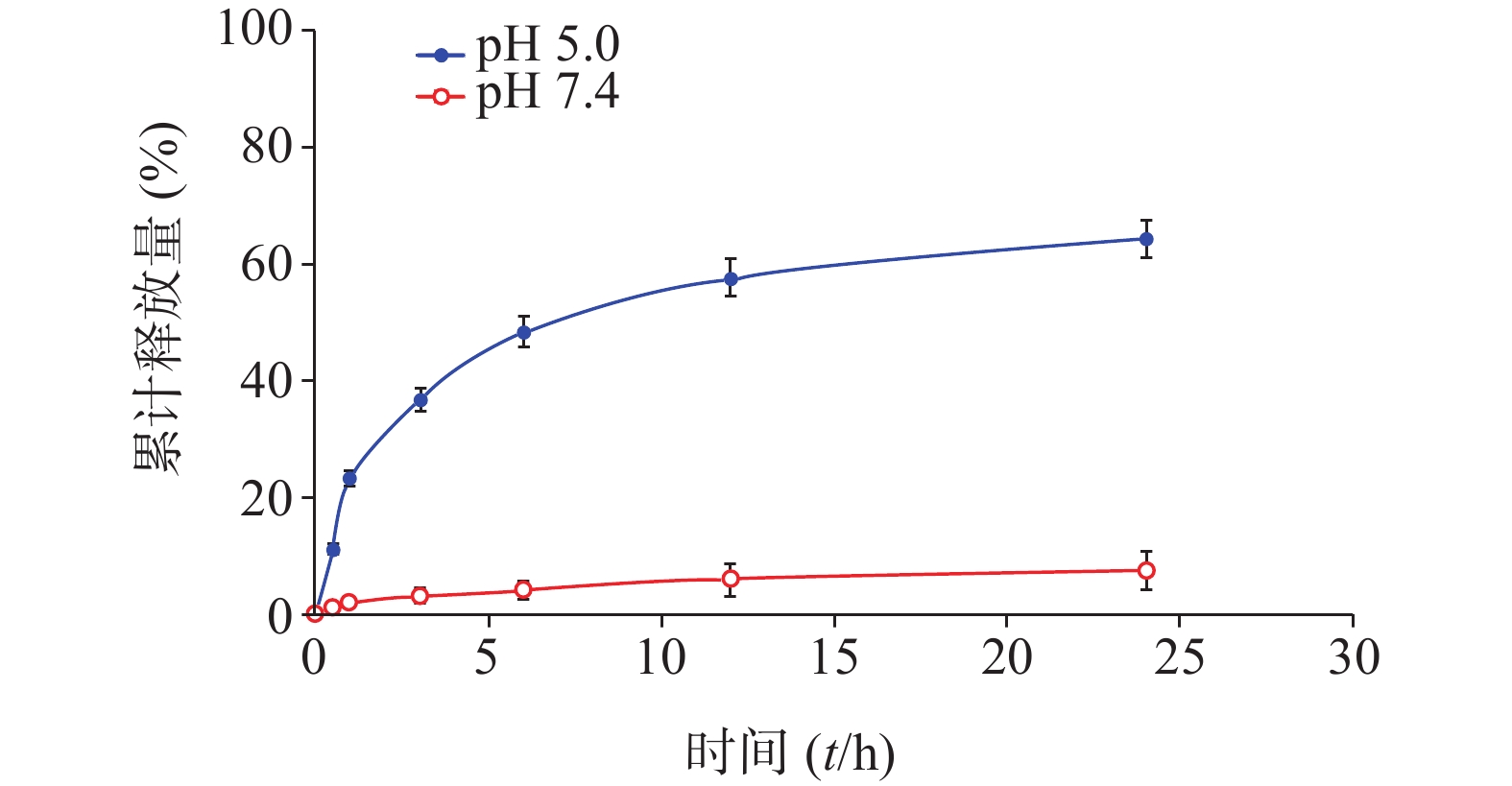
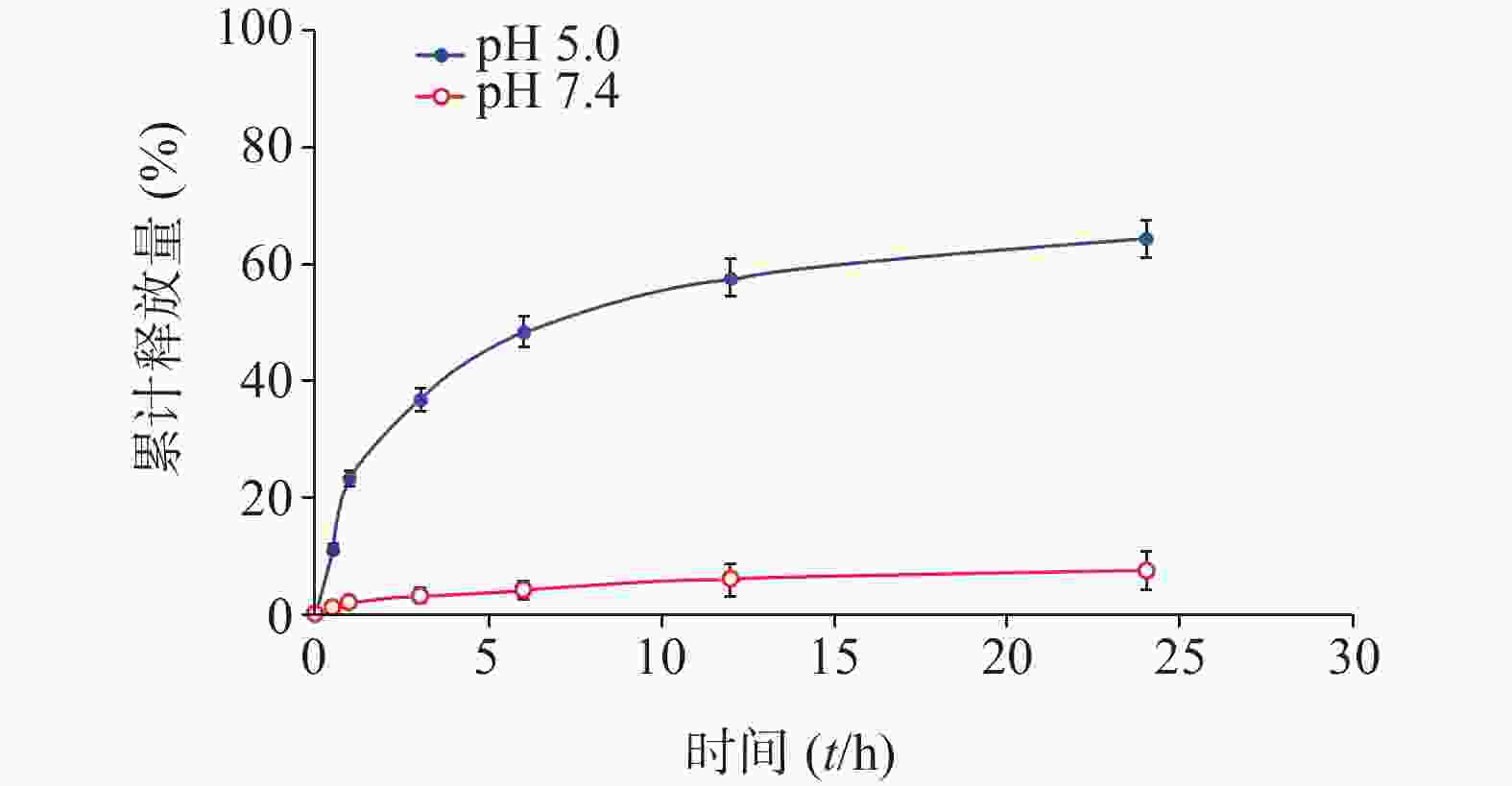
分别配制浓度为50 μmol/L的化合物1的HOAc-NaOAc缓冲液(pH 5.0)和PBS溶液(10 ml),并于0.5、1.0、3.0、6.0、12、24 h时分别取样(500 μl)。其中,HOAc-NaOAc缓冲液(pH 5.0)组取样液用0.1 mol/L氢氧化钠水溶液迅速调节pH值至7.4。每份取样液加PBS稀释至原溶液1/3浓度,微孔滤膜(孔径0.22 μm)过滤,HPLC进样检测;实验重复3次。根据5-Fu的HPLC峰面积-浓度标准曲线分析计算,绘制目标化合物1于弱酸(pH 5.0)中的5-Fu体外释放量-时间曲线,结果见图3。
-
参照文献[6-8]的方法,每孔5×103个B16-F10细胞或HepG2细胞悬液(100 μl)接种于96孔板上,加入等体积上述细胞培养液孵育24 h;更换含不同浓度待测物的培养液(DMSO浓度小于1%,100 μl),继续避光孵育48 h;再更换含10%(V/V)CCK-8(Beyotime,中国)的RPMI 1640基础培养基(100 μl),继续培养1.5 h,然后用Varioskan Flash全波长酶标仪(Thermo)于波长450 nm处测定每孔的吸光度值,计算各浓度对应的细胞存活率,并拟合得到待测物的肿瘤细胞半数抑制浓度即IC50值。
-
每孔5×103个B16-F10细胞或HepG2细胞悬液(100 μl)接种于96孔板上,加入等体积细胞培养液孵育24 h;更换含不同浓度待测物的细胞培养液(DMSO浓度小于1%,100 μl),继续避光孵育24 h;再更换新鲜培养液(100 μl),以波长为660 nm的激光辐照受试细胞样品(光照剂量为10 J/cm2),继续孵育24 h。最后按“2.3.2”项下CCK-8法测定各待测物的肿瘤细胞IC50值。
-
以临床光敏药物他拉泊芬为阳性对照,化合物1及其先导化合物3对肿瘤细胞株的体外PDT抗癌活性结果见表1。
化合物 B16-F10细胞 暗毒/光毒比 HepG2细胞 暗毒/光毒比 暗毒性 光毒性 暗毒性 光毒性 化合物 1 46.84±8.46*, ΔΔΔ 0.73±0.16**, ΔΔΔ 64.2 50.80±6.45**, #, ΔΔΔ 0.90±0.22**, ΔΔΔ 56.4 二氢卟吩e6 69.72±4.69 3.36±0.59 20.8 70.38±10.9 2.75±0.41 25.6 他拉泊芬 254.8±18.8 11.31±3.88 22.5 176.4±28.4 15.47±5.07 11.4 5-Fu 35.80±6.68 NTa − 39.16±2.7 NTa − NTa:未测定;*P < 0.05,**P < 0.01,与二氢卟吩 e6组比较;#P < 0.05,与5-Fu组比较;ΔΔΔP < 0.001,与他拉泊芬组比较。 -
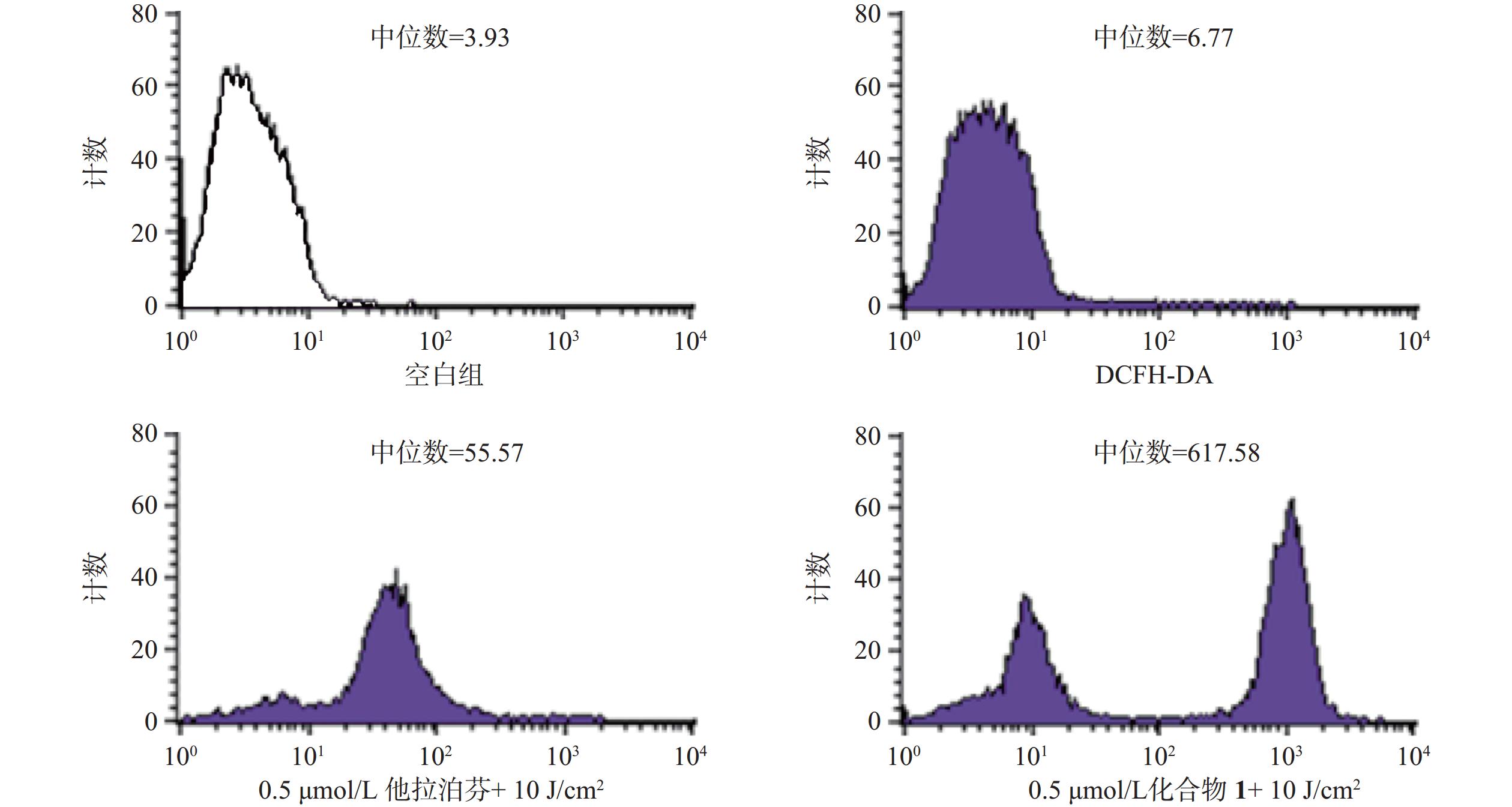
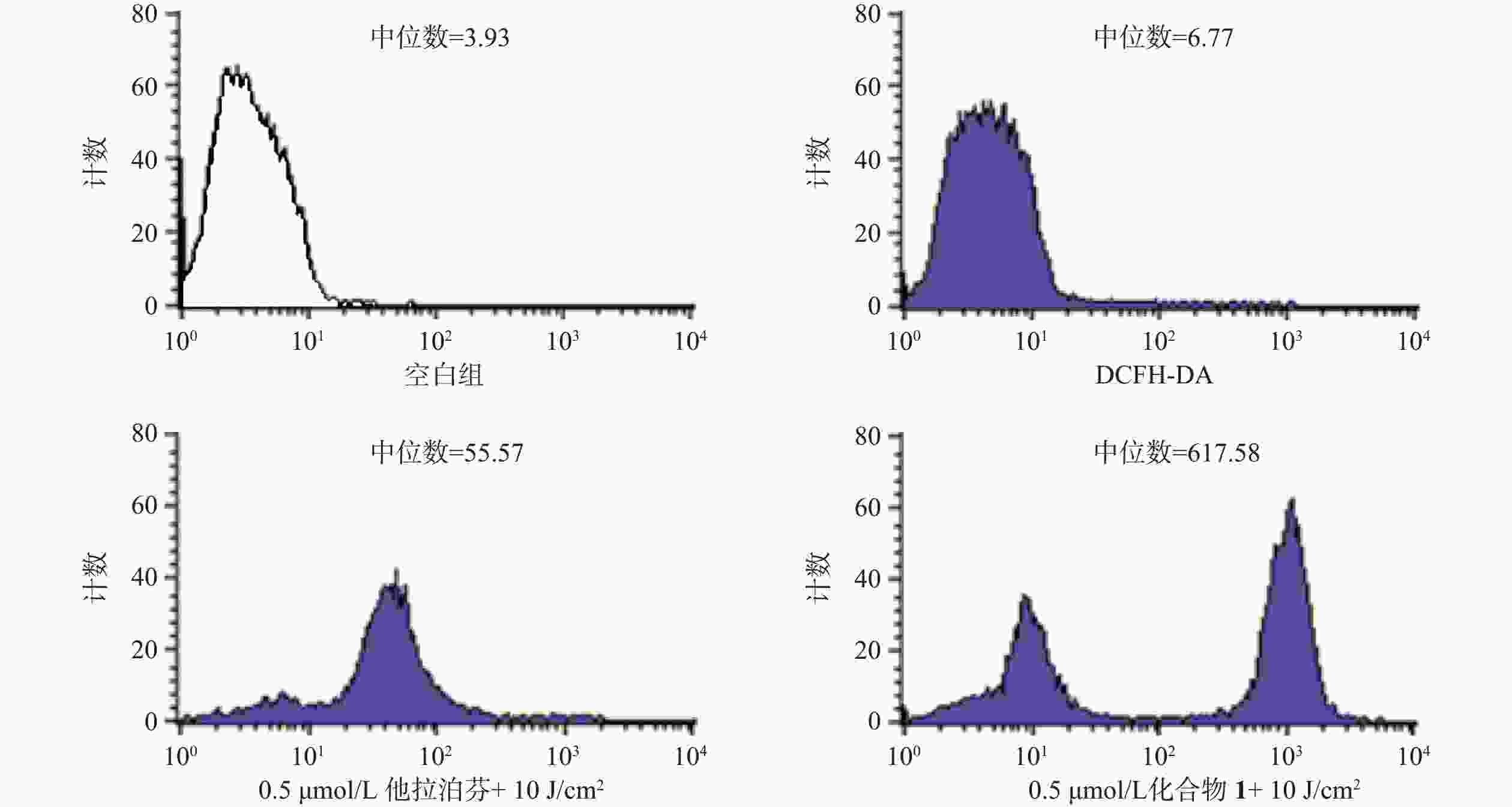
操作步骤如下:a. 每孔3 × 105个B16-F10细胞悬液(2 ml)接种6孔板上,按“2.3.1”项条件避光孵育24 h;b. 分别更换含一定浓度化合物1或他拉泊芬的新鲜培养液(DMSO浓度小于1%,2 ml),继续避光孵育24 h;c. 加入10 mmol/L DCFH-DAROS荧光检测探针(Beyotime,1.5 μl),吹打混匀,继续避光孵育20 min;d. PBS洗涤3次,再加新鲜培养液(2 ml),以660 nm波长的激光辐照(光剂量10 J/cm2)细胞样品,继续避光孵育20 min;e. 收集每孔细胞样品,用流式细胞仪检测各孔细胞ROS水平,结果见图4。
-
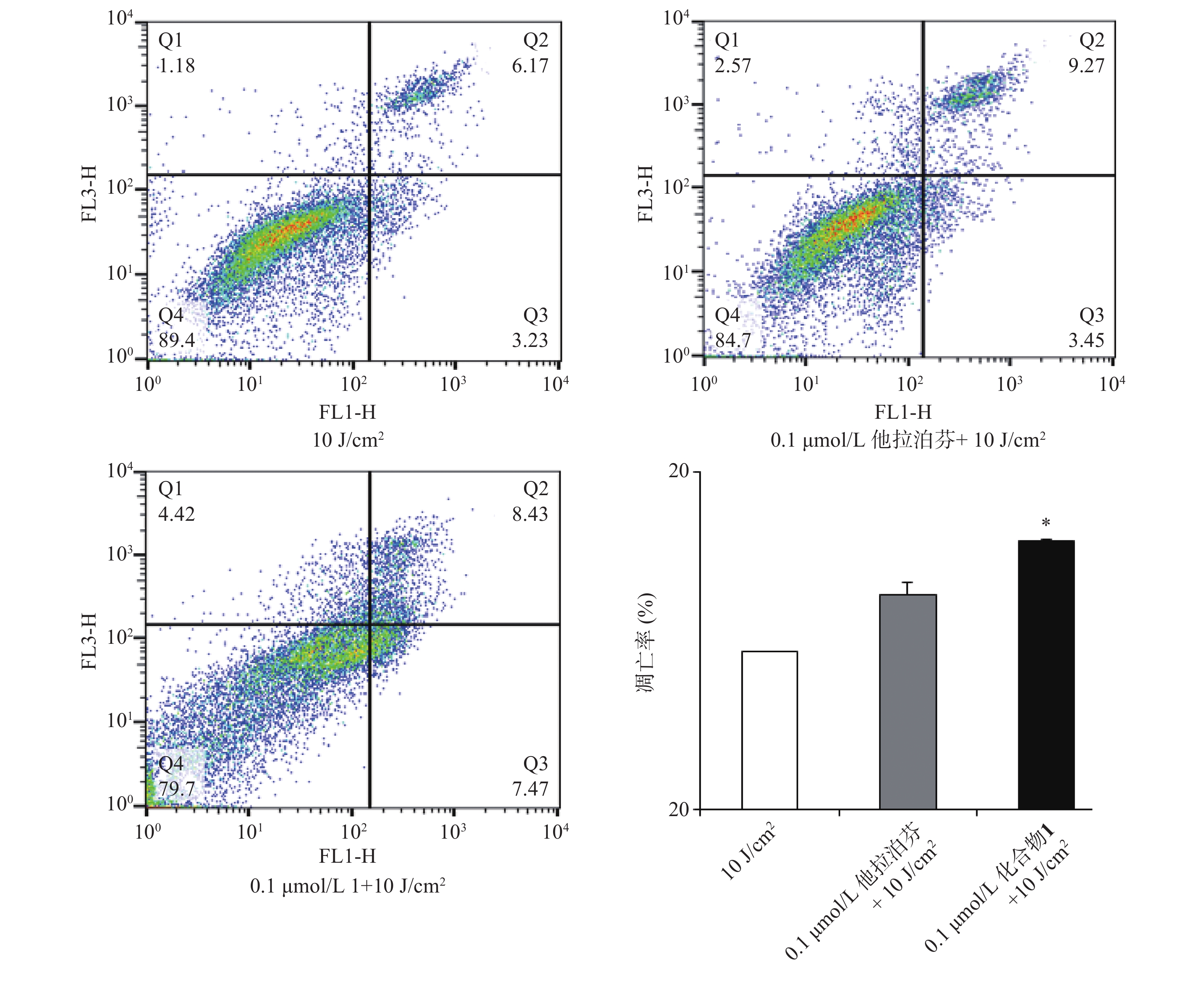
按“2.4”项下操作方法,仅从步骤c开始,更换新鲜培养液(2 ml),用660 nm波长的激光辐照(光剂量10 J/cm2)细胞样品,继续避光孵育20 min;d. 以1 500 r/min离心(5 min)细胞样品,PBS洗涤,再以1 000 r/min离心(5 min)后获取细胞样品;e. 按Annexin V-FITC细胞凋亡检测试剂盒(Beyotime)操作流程操作,结果见图5。
-
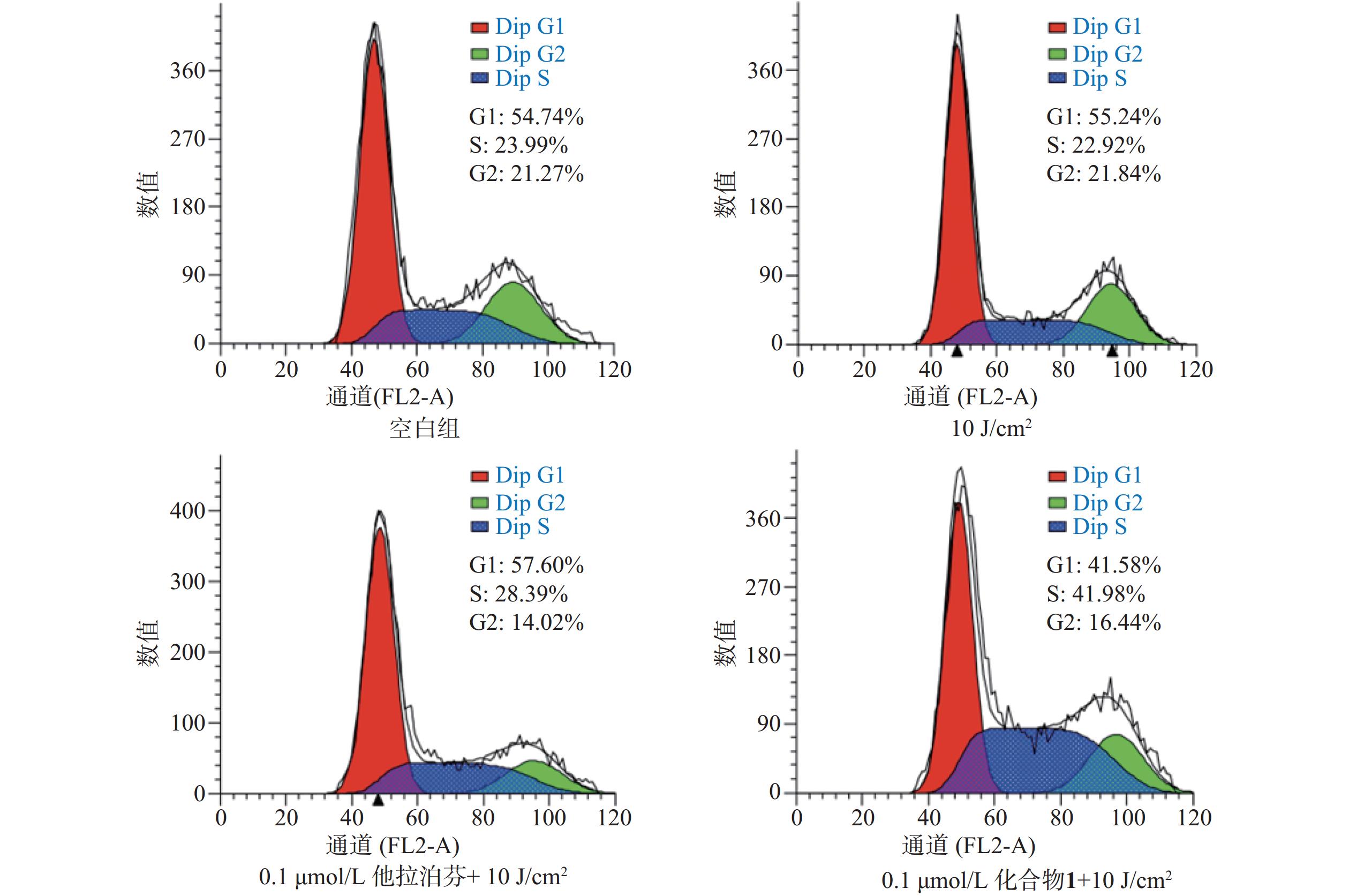
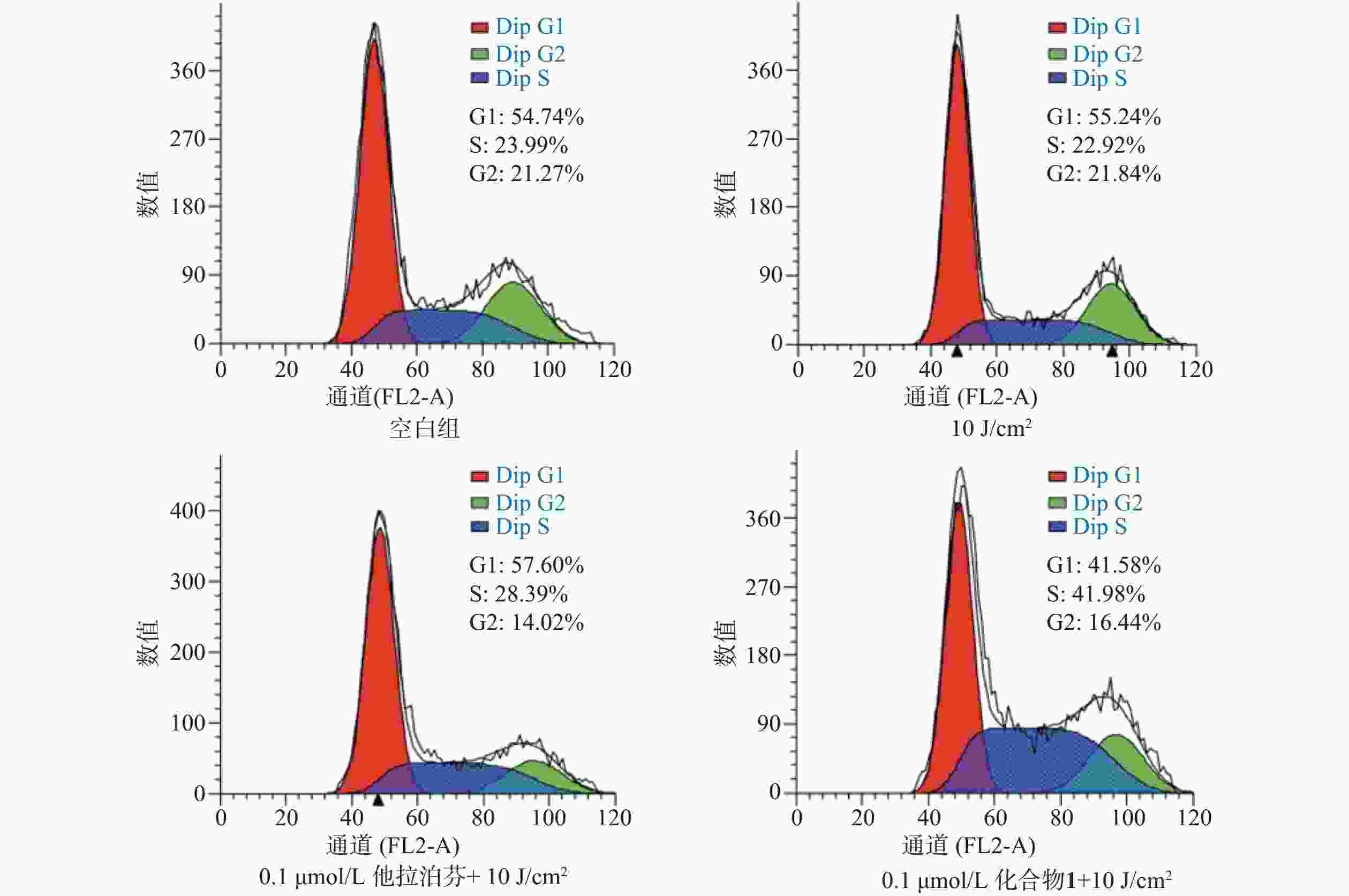
按“2.5”项下操作方法,仅在e步骤中,换以细胞周期阻滞检测试剂盒(Beyotime)的操作流程,每份细胞样品中分别加入染色缓冲液(300 µl)、RNase A(6 µl)和碘化丙啶染色液(15 µl),轻轻混匀,避光孵育20 min后,用流式细胞仪进行细胞周期阻滞检测,结果见图6。
-
按文献[14]方法制得的二氢卟吩e6(3)为先导化合物,经1-乙基-(3-二甲氨基丙基)碳二亚胺盐酸盐(EDC·HCl)于无水DMF中催化分子内脱水缩合制得二氢卟吩e6-131,152-酸酐活泼中间体[15],然后直接与中间体2发生酰化反应成功合成得到了光化疗双模抗肿瘤光敏剂二氢卟吩e6-偕氟尿嘧啶(1),反应收率达39.6%,其结构经UV、ESI-MS、1H NMR及元素分析确证。
化合物1在甲醇中最大紫外吸收波长和荧光发射波长(激发波长:400 nm)分别为660 nm和670 nm,与先导物3相一致,表明先导物3以酰腙键偶联5-Fu后,并没有改变其作为光敏剂特有的紫外吸收和荧光发射光谱等光物理特性。此外,化合物1在弱酸(pH 5.0)条件下,能有效释放5-Fu,24 h内累积释放率可达60.3%;但在pH 7.4的条件下较为稳定,24 h内5-Fu累积释放率仅为5%。
体外PDT抗癌活性测试结果显示,化合物1对B16-F10和HepG2细胞株的光毒活性和暗毒/光毒比(治疗指数)均显著优于先导物二氢卟吩e6(3)(P<0.005)和他拉卟吩(P<0.001),其IC50值分别达0.73 μmol/L和0.90 μmol/L。
体外PDT抗癌机制研究提示,化合物1介导的PDT能显著提升B16-F10细胞内ROS水平和诱导B16-F10细胞凋亡,并阻滞肿瘤细胞周期于S期。
总之,二氢卟吩e6-偕氟尿嘧啶(1)具有PDT抗癌活性强、治疗指数(暗毒/光毒比)高且可在肿瘤弱酸环境中有效释放5-Fu等优点,从而实现“单分子”光化疗协同抗肿瘤作用,值得进一步开发研究。
Synthesis and biological activities of chlorin e6-based conjugate of fluorouracil as dual-mode antitumor photosensitizer
doi: 10.12206/j.issn.2097-2024.202306030
- Received Date: 2023-06-14
- Rev Recd Date: 2023-11-07
- Available Online: 2024-01-19
- Publish Date: 2024-01-25
-
Key words:
- synthesis /
- photodynamic therapy /
- photosensitizer /
- chlorin e6 /
- fluorouracil (5-Fu) /
- antitumor
Abstract:
| Citation: | SHEN Jie, HUANG Fei, ZHANG Xingjie, YAO Jianzhong. Synthesis and biological activities of chlorin e6-based conjugate of fluorouracil as dual-mode antitumor photosensitizer[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(1): 18-23. doi: 10.12206/j.issn.2097-2024.202306030 |








 DownLoad:
DownLoad: