-
细菌感染是一类高发病率和高致死率疾病,而细菌耐药给抗细菌感染治疗带来了严峻挑战,预计到2050年,耐药细菌感染将导致全球上千万人死亡[1]。抗生素作为首选药物直接杀灭细菌对细菌带来“选择性压力”,即耐药现象,它将伴随着抗生素在临床的使用而广泛发生[2]。因此,亟需设计和研发新型的给药策略以改善细菌乃至耐药菌感染的预防和治疗效果。
创伤弧菌(Vibrio Vulnificus),是一种嗜盐性G−菌,广泛存在于亚热带和热带海域,在我国浙江、福建和广东沿海海域均有分布。伤口感染后表现为皮肤坏死、筋膜炎和坏疽,严重感染者需大面积清创甚至截肢。更加严重的会引起原发性败血症,严重败血症患者的病死率超过了50%[3]。全球范围内创伤弧菌对多种抗菌药物存在不同程度的耐药性,如在中国海域创伤弧菌对链霉素、庆大霉素和头孢唑啉的耐药性分别达45.45%、93.94%和100%[4]。研发防治创伤弧菌感染新型疗法对于控制感染具有重要意义。创伤弧菌溶血毒素A(VvhA)是创伤弧菌向胞外释放的唯一外毒素,也是成孔毒素CDC家族中的一员,为胆固醇依赖型成孔毒素,是引发细胞、组织损伤的重要毒力因子。成孔毒素在细菌感染的发病机制中发挥了重要作用,VvhA通过在细胞膜上低聚形成有效直径约为1 nm的小孔,导致胶体渗透使细胞溶解[5]。目前,毒力因子靶向疗法已成为抗生素耐药感染的替代疗法。阻断毒素不仅能避免其对机体产生的严重损害并能阻碍细菌在体内的存活。这种抗毒力因子的方式并非直接杀灭细菌,且不易发生耐药现象[6]。体外研究已经阐明了VvhA对上皮细胞、脐静脉内皮细胞、巨噬细胞和淋巴细胞等均具有一定的细胞毒性[7]。Qin等发现VvhA可以触发巨噬细胞RAW264.7的炎症反应[8]。2013年,Zhang等提出“纳米海绵”的载体系统,能有效吸附金黄色葡萄球菌的成孔毒素从而起到解毒作用[9]。本研究将巨噬细胞膜和人工脂质体杂合,以VvhA为成孔毒素模型,制备了巨噬细胞脂质体杂合载体并进行表征,考察巨噬细胞膜杂合脂质体的体内外解毒能力,为进一步创伤弧菌感染的抗毒素治疗提供实验依据。
-
胎牛血清(澳洲EallBio公司);RPMI1640培养基(美国Gibco公司);DMEM培养基(美国Hyclone公司);卵磷脂(艾伟拓医药科技有限公司);DSPE-mPEG2000(Laysan Bio公司);CCK-8试剂盒(北京索莱宝科技有限公司);脂质体挤出器(美国Avanti Polar Lipids公司);Synergy 2酶标仪(美国Bio-Tek公司);马尔文粒度电位仪(英国Malvern);R206D旋转蒸发仪(上海申生公司);H2050R-1台式高速冷冻离心机(湖南湘仪公司)。VvhA毒素蛋白由本实验室合成。小鼠巨噬细胞Raw 264.7;人结肠上皮细胞(NCM460)(中科院上海细胞库);BALB/c小鼠(雌性,6~8周龄,体重18~20 g,上海斯莱克实验动物有限责任公司),动物实验经过海军军医大学实验委员会的批准,并按照动物伦理学进行。
-
用DMEM高糖培养基,10% FBS,5% CO2,温度37 ℃正常培养Raw 264.7巨噬细胞。参考文献方法提取巨噬细胞膜[10]:细胞刮刀获取细胞,4 ℃预冷的TM缓冲液洗涤细胞2次并重悬细胞,使浓度约为2.5×107细胞/ml。将细胞悬液置于−80 ℃,反复冻融3轮,探头超声破碎细胞(5 min,功率44 W)得到细胞匀浆,加入1 mol/L的蔗糖溶液,4 ℃ 2 000 g离心10 min去除细胞核和未裂解细胞,上清4 ℃ 3 000 g离心30 min以收集细胞膜。细胞膜用含有0.25 mol/L蔗糖的TM-buffer重悬液,4 ℃ 3 000 g离心1 h洗涤,离心收集PBS重悬液,−80 ℃保存。
-
采用薄膜水化-挤出法制备巨噬细胞膜杂合脂质体(MM-PLs)[11]。将蛋黄卵磷脂(EPC)、胆固醇(CHO)和DSPE-mPEG2000以4∶4∶1的摩尔比用二氯甲烷溶解完全,加入100 ml茄形瓶中,室温水浴进行旋转蒸发去除二氯甲烷,使脂质材料在茄形瓶壁中成膜,完全去除二氯甲烷后加入适量巨噬细胞膜和纯水水化,水化后冰浴超声5 min,脂质体挤出器分别连续挤出通过400、200、100 nm聚碳酸酯膜各20次以制备MM-PLs。
对照组巨噬细胞膜囊泡(MMVs)和脂质体(PLs)的制备同巨噬细胞膜杂合脂质体。将适量巨噬细胞膜悬液室温水化1 h,水浴超声和挤出后得到MMVs。脂质体的制备为薄膜水化法,材料方法同上。
-
马尔文粒径仪测定MM-PLs、MMVs、PLs的粒径和Zeta电位。将一定浓度的MM-PLs滴加到碳支持膜(铜网)上,待晾干后再滴加醋酸铀水溶液(1%,W/V)进行负染,透射电镜(TEM)观察形态。
-
BALB/c小鼠(雌性16~18 g,6~8周龄),用3%异氟烷麻醉2~3 min后下颌下静脉抗凝取血,低温离心5 min(300 g),弃上清液后加入PBS重悬,得到小鼠红细胞,PBS稀释成2.5%红细胞悬液(V/V),4 ℃保存备用。
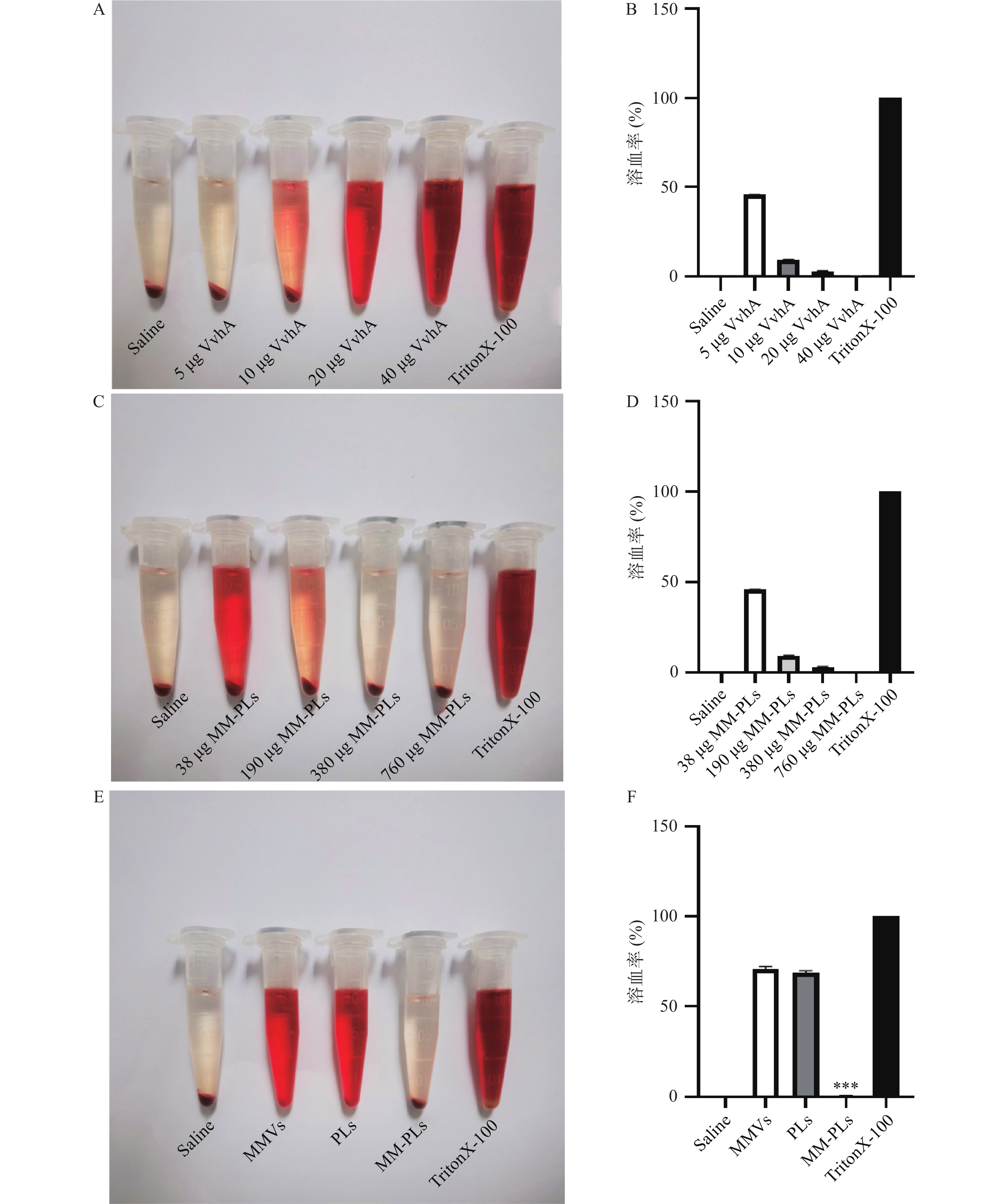
分别取VvhA(2 mg/ml)5、10、20、40 μg,各加入相应量的PBS定容,再加入1 ml 2.5%红细胞悬液混匀,置于37 ℃孵育1 h,室温离心5 min(2 000 g)后取上清液,1×PBS 1∶1稀释后用酶标仪测定其在540 nm处的A值(n = 3)。取40 μg VvhA,分别加入不同MM-PLs的量(38、190、380、760 μg),重复上述溶血实验(n=3),PLs和MMVs作为对照组,考察MM-PLs对毒素吸附的量效关系。TritonX-100组作为阳性对照(100%溶血),PBS组作为阴性对照(0%溶血)。根据以下公式对各个样品的溶血程度计算溶血率:
-
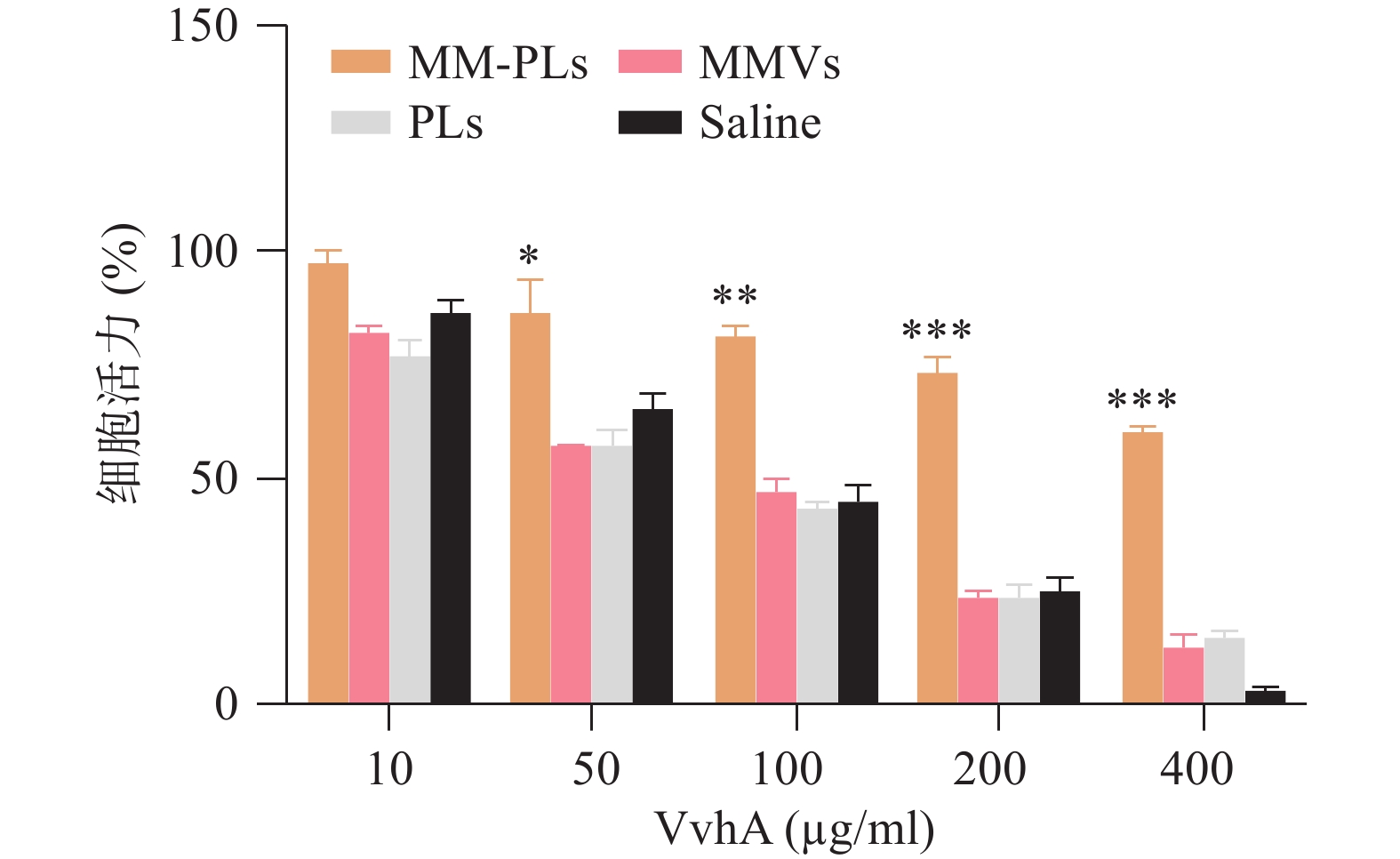
文献表明VvhA对上皮细胞有一定的细胞毒性[7]。本实验采用结肠上皮细胞(NCM460)为细胞模型,CCK-8法测定细胞活力,以研究MM-PLs的细胞水平解毒能力。NCM460按5 000个/孔接种于96孔板,37 °C、5% CO2培养过夜。将50 μl MM-PLs、PLs、MMVs和生理盐水分别与不同浓度的VvhA(10、50、100、200和400 μg/ml)室温孵育3 h。而后加入96孔板中,与NCM460在37 ℃下共孵育12 h。孵育完毕后每孔加入10 μl CCK-8溶液,继续37 ℃下孵育1 h,酶标仪测量450 nm处OD值,计算细胞存活率。
-
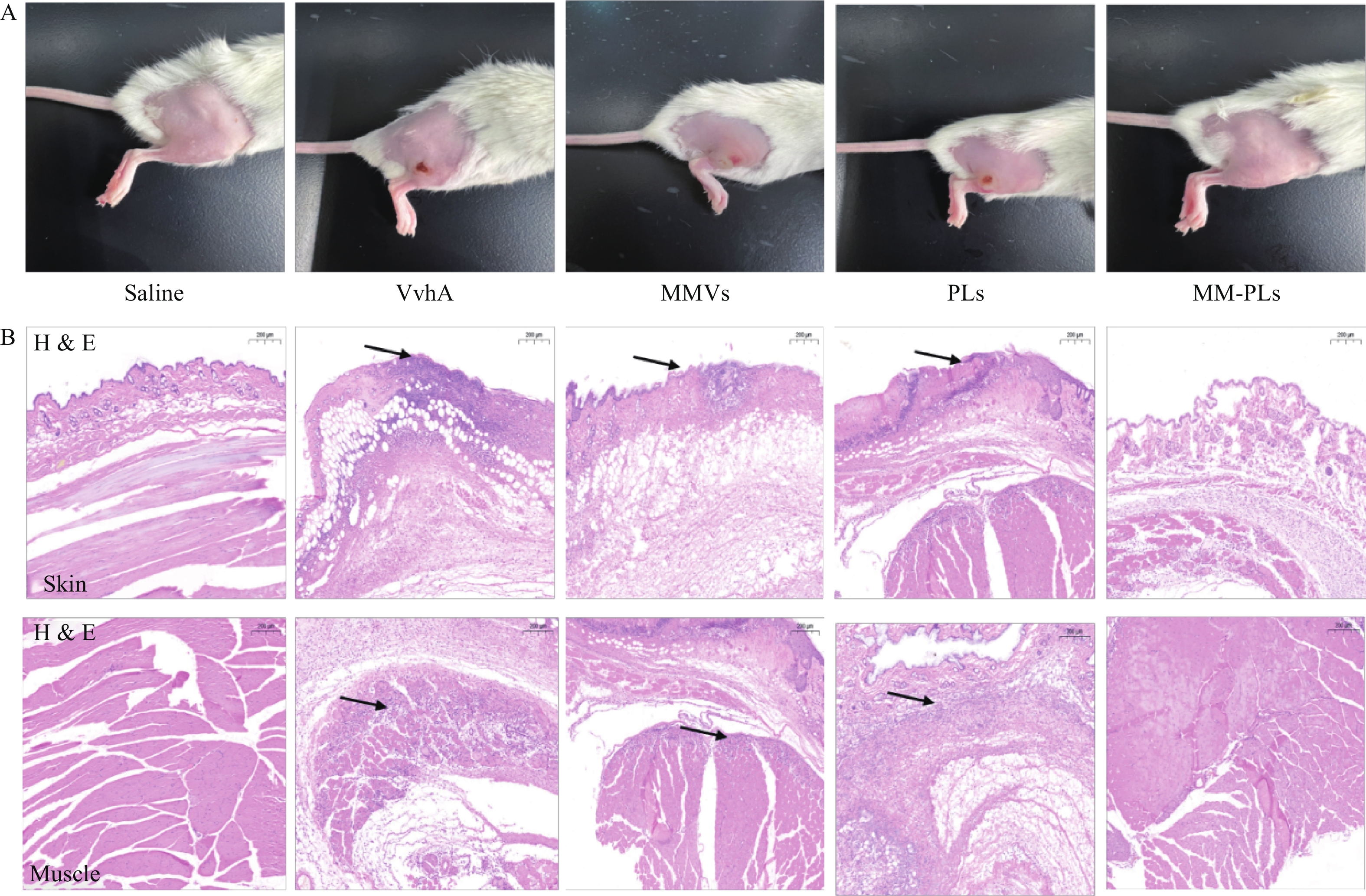
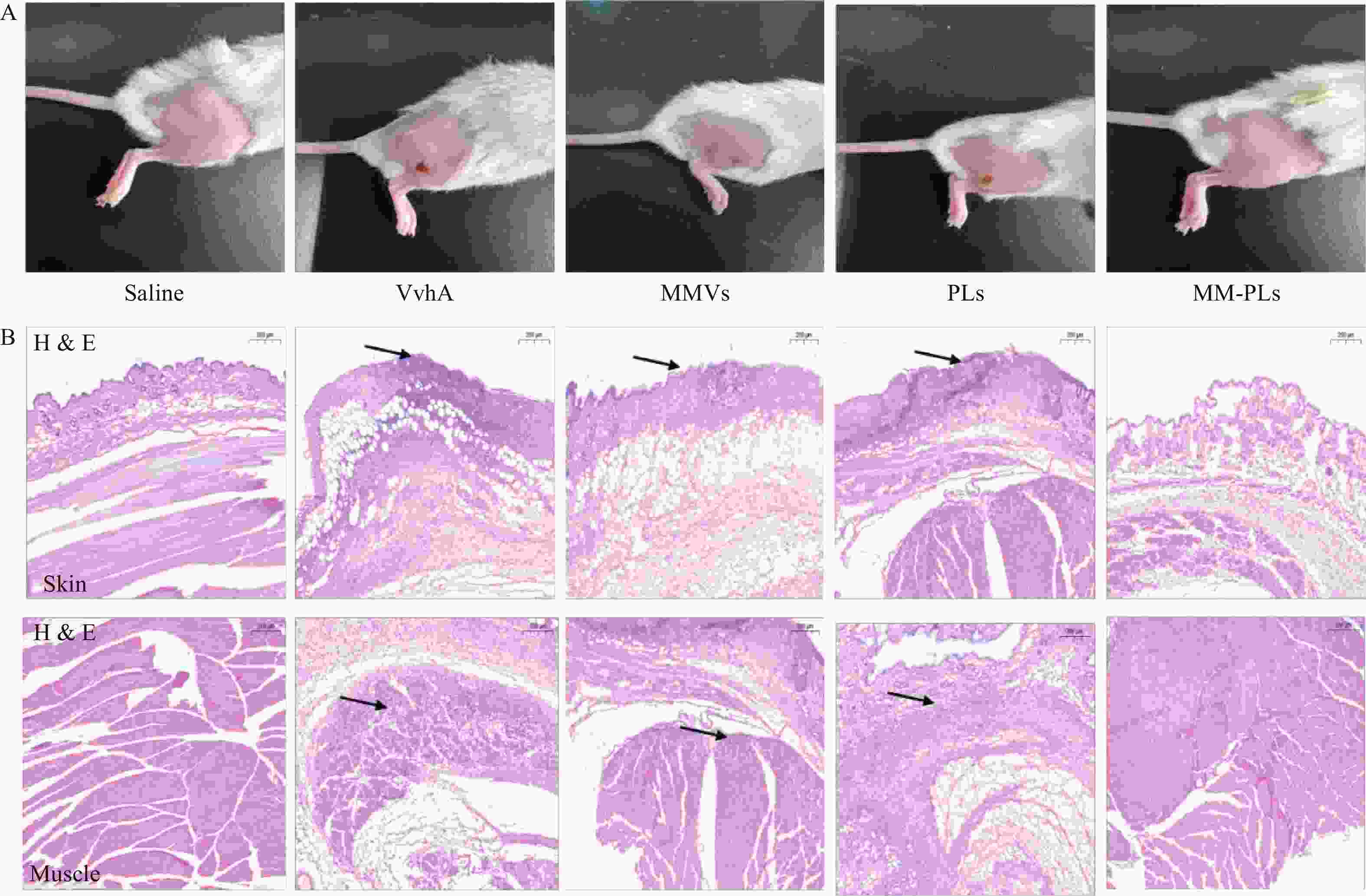
取BALB/c小鼠(雌性,18~20 g,6~8周龄)30只,随机分为5组(生理盐水组、VvhA组、PLs组、MMVs组和MM-PLs组)。小鼠后肢脱毛处理,生理盐水组为阴性对照,实验组将80 μg VvhA毒素皮下注射至后肢,再分别将生理盐水、PLs、MMVs和MM-PLs在同一部位皮下注射,观察皮肤组织的溃烂愈合情况。72 h后处死小鼠,取注射部位的皮肤和肌肉,用4%多聚甲醛固定,石蜡包埋切片后进行H&E染色。
-
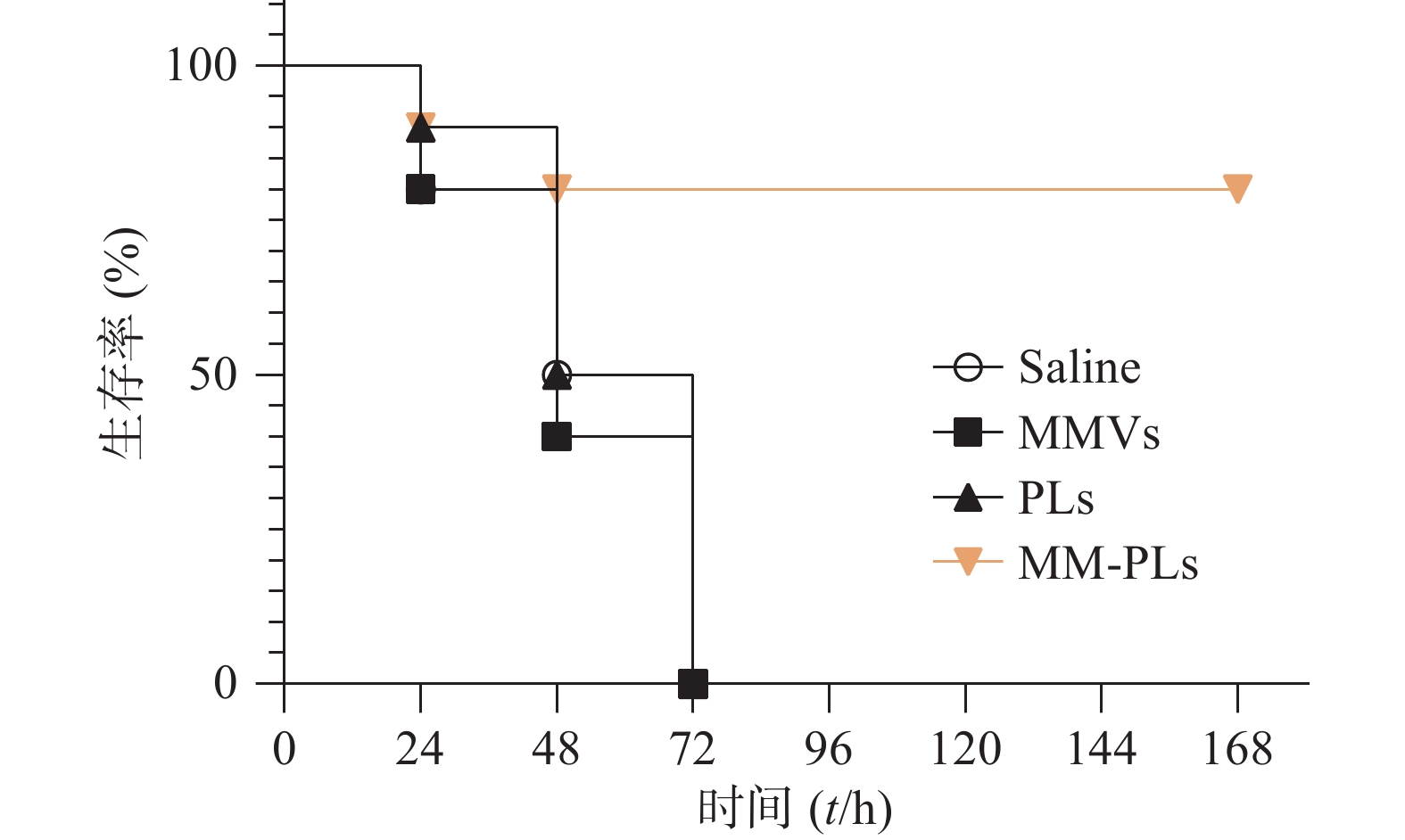
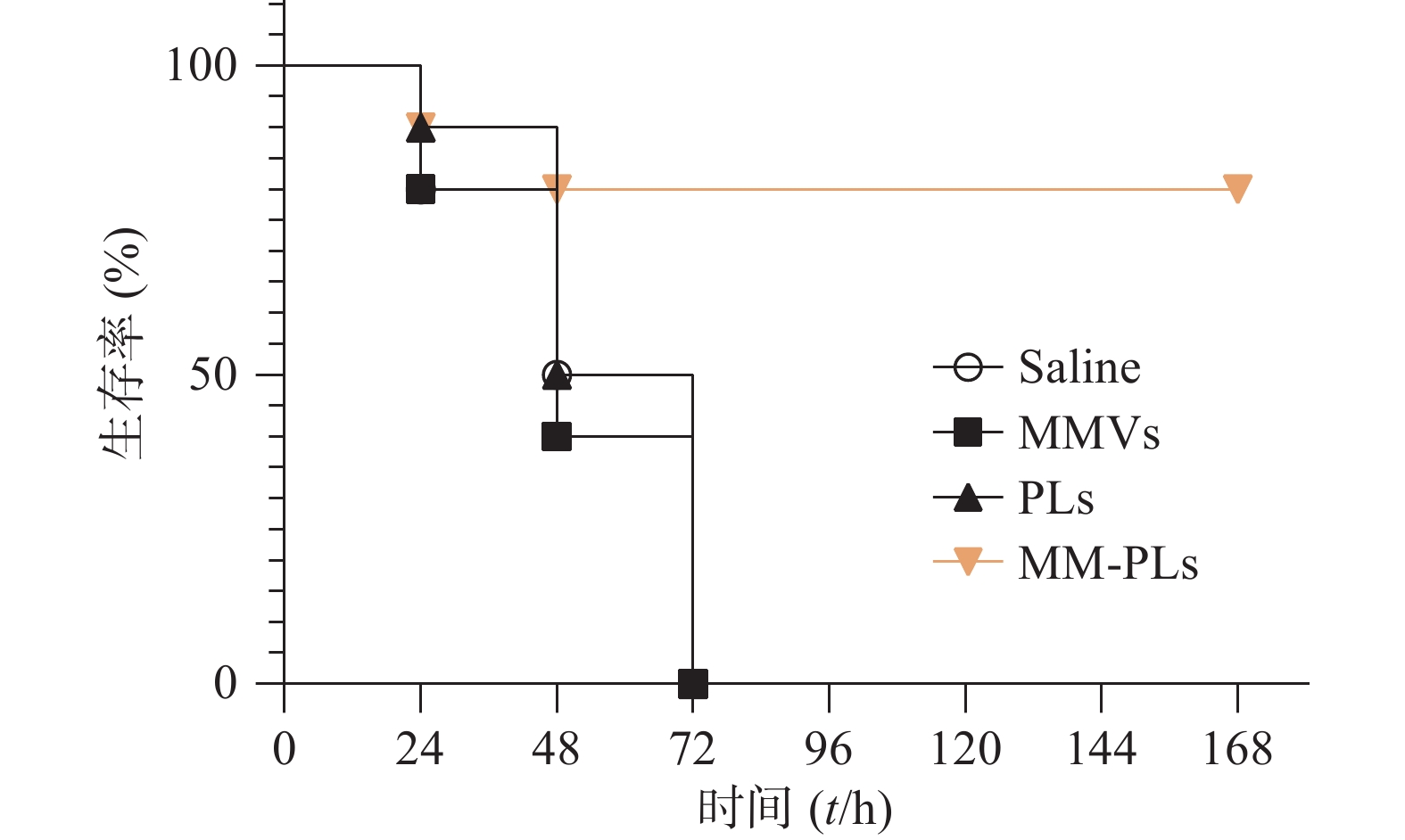
取BALB/c小鼠(雌性,18~20 g,6~8周龄)40只,随机分为4组(VvhA组、PLs组、MMVs组和MM-PLs组)。小鼠腹腔注射致死剂量的VvhA(10 mg/kg),5 min后分别腹腔注射一定剂量的生理盐水、PLs、MMVs和MM-PLs。观察各组小鼠精神状态及进食情况,并记录小鼠生存率。
-
采用GraphPad Prism 8.2统计学软件进行统计分析。统计方法采用独立样本t检验,P<0.05表示差异有统计学意义。
-
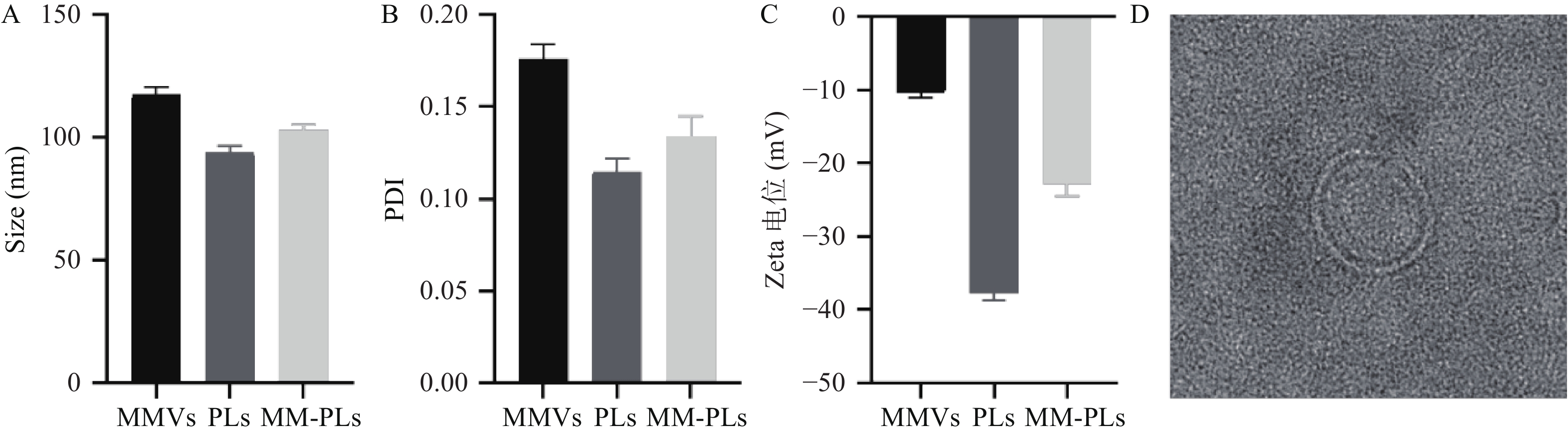
MMVs平均粒径为120.6 nm,PLs的平均粒径为93.9 nm,MM-PLs的平均粒径为101.1 nm(图1A),MMVs、PLs和MM-PLs的分布均较窄(图1B)。在杂合巨噬细胞膜后,MM-PLs Zeta电位值(−22.9 mV)介于MMVs(−10.4 mV)与PLs(−37.7 mV)之间(图1C)。TEM结果显示,MM-PLs呈规则类球形,大小均一,粒径约100 nm(图1D),与PLs的形态、大小类似,说明巨噬细胞膜的加入对脂质体的纳米微粒性质无显著影响。
-
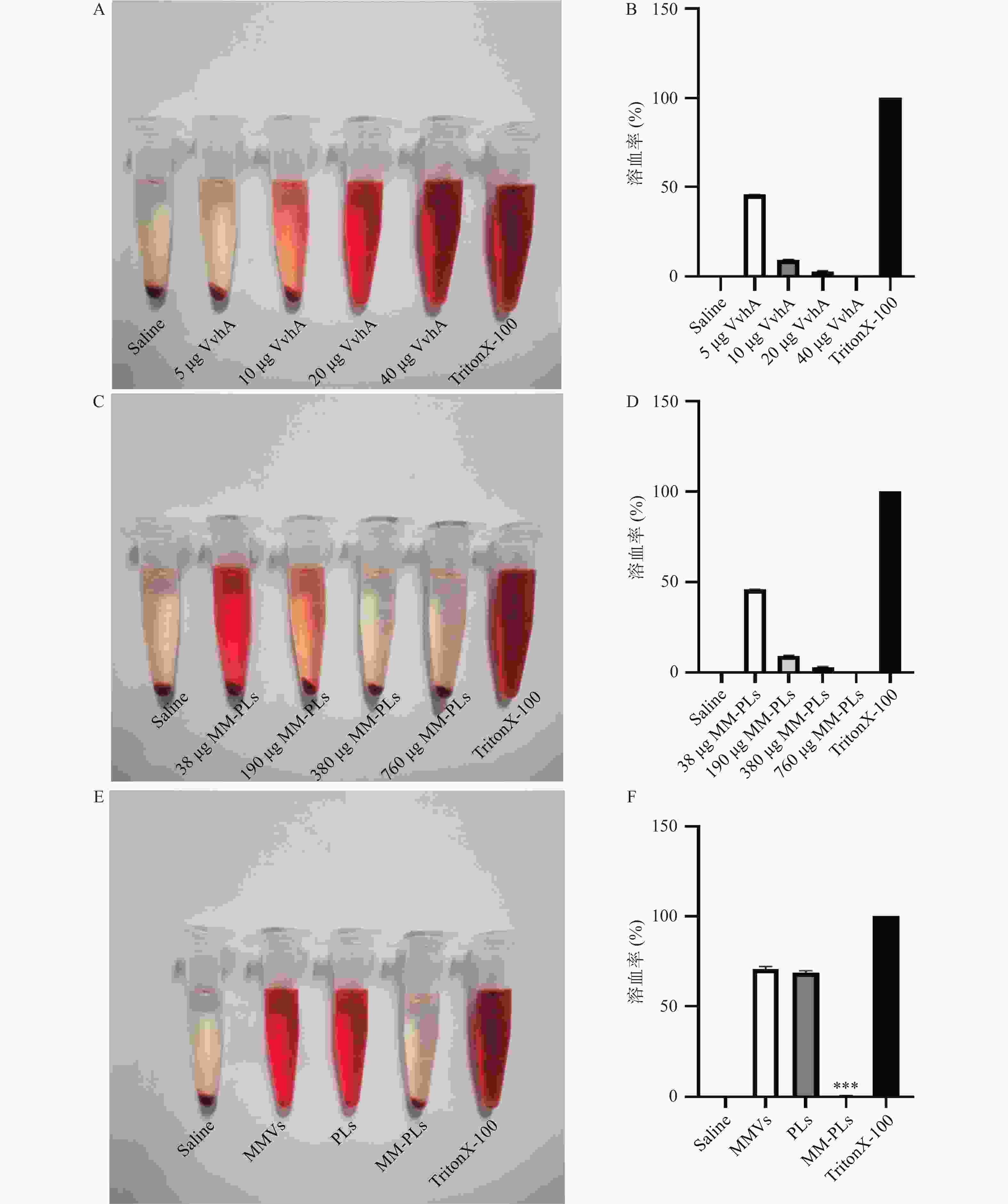
结果显示,40 μg的VvhA可以使1 ml 2.5%红细胞悬液完全溶血;380 μg的MM-PLs几乎可以完全中和40 μg的VvhA毒素蛋白,抗溶血率达到97.03%,而PLs和MMVs组均产生溶血现象,无中和毒素能力(图2)。
-
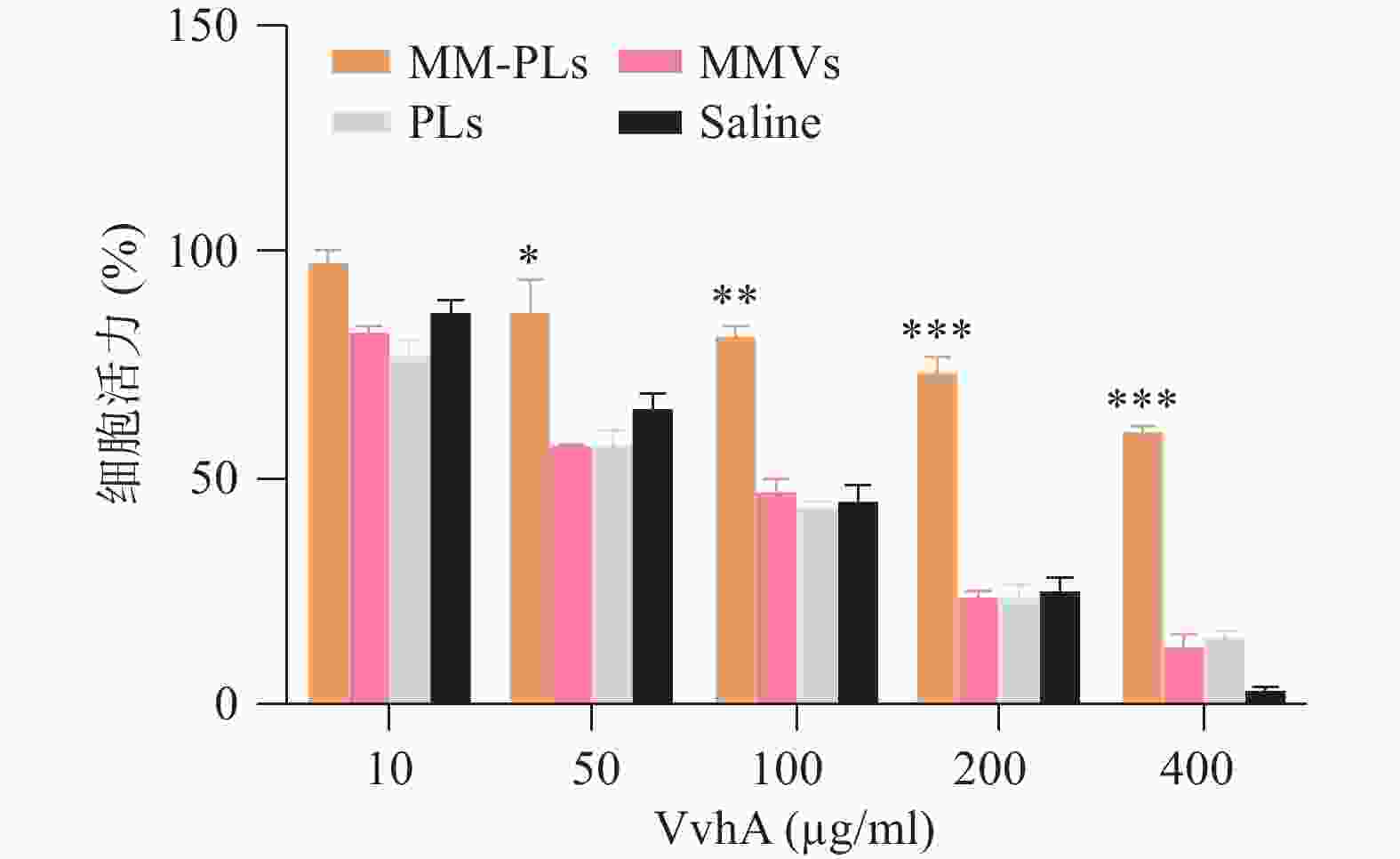
结果显示,细胞活力与VvhA呈剂量依赖性,与对照组相比MM-PLs组显著提高细胞活力,具有一定的毒性抑制作用,而PLs组和MMVs组也具有一定的毒素吸附能力,但随着毒素浓度的增加,这种毒素中和能力显著降低(图3)。
-
结果显示(图4),阴性对照生理盐水组注射部位皮肤光滑完整,阳性对照组VvhA组注射部位有明显的皮肤组织溃烂, MMVs组和PLs组与阳性对照组情况类似,有面积相近的皮肤溃烂;MM-PLs组皮肤无明显损伤。H&E染色结果表明,VvhA组,VvhA+MMVs组和VvhA+PLs组的皮肤和相邻肌肉组织结构有明显炎症细胞浸润及组织细胞坏死凋亡现象,而VvhA+MM-PLs组组织结构完整,无炎症浸润及凋亡发生。
-
生理盐水组、MMVs组和PLs组小鼠在给予VvhA 6 h后陆续出现精神萎靡、被毛竖立、食欲减退、呼吸困难等症状,在注射毒素后 72 h内陆续死亡,而MM-PLs组小鼠在给予毒素后 24 h内死亡1只,48 h内共死亡2只,其余全部存活,存活率为80%(图5)。巨噬细胞膜杂合脂质体有较好的中和毒素效果,对模型动物产生保护作用。
-
细菌感染过程中,释放的毒力因子(如成孔毒素)对机体有较大的毒性作用[12]。因此,中和细菌毒素能够抑制细菌的存活与繁殖,同时减少其对组织的损伤[13]。抗毒素治疗针对细菌分泌的毒力因子,不直接对细菌产生杀伤作用,因而不易产生耐药现象[14]。不同的成孔毒素结构不一,但有相似的细胞膜作用机制,通过与细胞膜表面的特异性受体结合,聚集形成孔道,从而改变细胞的通透性并引发毒性[15]。本文将巨噬细胞膜与脂质体结合,既充分利用巨噬细胞作为VvhA毒素蛋白的天然靶点,其细胞膜具有特有的毒素捕获能结合能力,又发挥了人工脂质体扩大毒素聚集于载体膜的空间优势。PEG修饰实现纳米载体表面“隐形”修饰,避免RES系统的捕获,增加循环的稳定性。所制备的杂合载体能够中和VvhA,抵抗毒素的溶血作用和细胞毒性作用,我们也发现未经杂合的巨噬细胞膜囊泡和脂质体也有一定的抑制细胞毒性作用(一定的抗溶血性),推测巨噬细胞膜可吸附部分毒素,但无脂质有效扩大毒素的吸附空间,而有研究表明,磷脂双分子层具有蛋白质可插入特性[16],因此脂质体也可吸附少量毒素,但是将细胞膜与脂质体杂合能有效地提高毒素的结合。文献表明, VvhA对上皮细胞、肝脏细胞、内皮细胞均具有一定的毒性[17],细胞毒实验结果表明所构建的杂合载体能够有效吸附并中和毒素的毒性,减少细胞毒性。而小鼠皮肤解毒、小鼠注射毒素后的生存曲线结果均表明经过杂合载体毒素的吸附结合,在体内能够很好的中和VvhA毒性,对动物具有抵抗类毒素的保护作用。
Preparation and anti-Vibrio vulnificus hemolysin A of macrophage membrane hybrid liposome
doi: 10.12206/j.issn.2097-2024.202207001
- Received Date: 2022-07-01
- Rev Recd Date: 2022-10-09
- Available Online: 2023-07-14
- Publish Date: 2023-01-25
-
Key words:
- Vibrio vulnificus /
- hemolysin A /
- biomimetic nanocarrier /
- bacterial infections
Abstract:
| Citation: | DAI Yu, GUO Lingyi, WANG Hongbo, BIAN Kangqing, YU Yuan. Preparation and anti-Vibrio vulnificus hemolysin A of macrophage membrane hybrid liposome[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(1): 26-30, 55. doi: 10.12206/j.issn.2097-2024.202207001 |







 DownLoad:
DownLoad: