-
泰山白首乌来源于为萝摩科(Asclepiadaceae)鹅绒藤属(Cynanchum Linn.)植物戟叶牛皮消Cynanchum bungei Decne. 的干燥块根,《本草备要》记载:“具有养血补血、补肝肾、强筋骨和润肠通便的作用”,也被誉为泰山四大名药之首[1-2]。研究表明,泰山白首乌的主要活性成分为苯乙酮和C21甾体皂苷[3-7]。泰山白首乌的主要药理活性有抗肿瘤、保肝、抗炎、抗菌、抗病毒、抗抑郁、降血糖 等[5-7]。
植物内生菌是一类广泛存在于宿主植物体内,且不引起宿主明显病症的真菌,是一类具有丰富多样性的微生物类群。植物内生菌通过“协同进化”作用,以促进宿主植物生长,增强抗逆性,促进药用植物中有效成分的积累[8-9],植物内生菌已成为国内外学者的研究热点。顾晓洁等[10] 2018年报道了滨海白首乌块根中内生细菌的分离鉴定。Li等[11]从滨海白首乌中分离出的一株产红色素具有抗氧化作用的内生真菌Stemphylium lycopersici。Gu等[12]从滨海白首乌中分离得到的内生真菌Plectosphaerella cucumerina YCTA2Z1中分离鉴定得到13种化合物,分离得到与宿主滨海白首乌相同的次级代谢产物单体告达庭(caudatin)、白首乌二苯酮、cynandione B和 2',5'-二羟基苯乙酮[11-12]。但是,目前没有关于泰山白首乌内生真菌的传统分离纯化培养报道。同时,有研究表明,泰山白首乌的粗提物和单体化合物对多种肿瘤细胞株均具有显著活性[6],目前已有从植物中分离得到具有抗肿瘤活性的内生真菌[13-14]的研究,但对泰山白首乌内生真菌的相关分离鉴定、活性成分及抗肿瘤等生物活性的研究还未开展。
本实验以泰山白首乌内生真菌为研究对象,通过传统分离培养法,将分离鉴定得到的泰山白首乌内生真菌进行液体发酵,并进行抗肿瘤活性菌株筛选。一方面探讨泰山白首乌内生真菌能否产生与宿主相似的次级代谢产物,另一方面为研发新的抗肿瘤活性药物提供科学依据。
-
3个产地的健康泰山白首乌植株各5株,包含根、茎、叶。济南的泰山白首乌叶(JTY)、泰山白首乌茎(JTJ)、泰山白首乌根(JTG),均采自山东中医药大学长清校区植物园内(36°56′ N, 116°79′ E);临沂的泰山白首乌叶(LTY)、泰山白首乌茎(LTJ)、泰山白首乌根(LTG),均采自临沂费县御华景宸农业生态园内(35°27′ N, 117°97′ E);泰安的泰山白首乌叶(TTY)、泰山白首乌茎(TTJ)、泰山白首乌根(TTG),均采自泰山(35°78′ N, 117°45′ E)。植物样品经山东中医药大学中药鉴定教研室徐凌川教授鉴定为泰山白首乌C. bungei Decne.。采集的样品用无菌塑料袋包装,置于4 ℃冰箱保存备用,48 h内进行样品处理。
-
T100™梯度PCR扩增仪(美国BIO-RAD伯乐T100梯度PCR仪);电泳仪(上海天能科技有限公司);凝胶成像仪(上海天能科技有限公司);Qubit® 2.0荧光计(赛默飞Invitrogen);SW-CJ-1D超净工作台(苏州净化设备有限公司);E.Z.N.A.真菌DNA提取试剂盒(美国Omega Bio-Tek);Taq DNA Polymerase(赛默飞Thermo);Agencourt AMPure XP(Beckman);2×Trans Taq High Fidelity (HiFi) PCR SuperMix I(北京全式金生物技术有限公司);ddH2O(北京全式金生物技术有限公司);琼脂糖(超纯)(北京天根生物科技有限公司);50×TAE缓冲液(北京索莱宝科技有限公司Solarbio);DNA Maker(日本TaKaRa);Goldview核酸染料(10 000×);6×Loading buffer(日本TaKaRa);XD-101 CO2细胞培养箱(日本SANYO公司);奥林巴斯IX51倒置荧光显微镜(日本奥林巴斯公司OLYMPUS);ELX800光吸收酶标仪(美国BioTek);细胞培养瓶(美国FALCON);青、链霉素混合液(北京索莱宝科技有限公司);PBS(北京索莱宝科技有限公司);RPMI-1640(美国GIBCO);DMEM(美国GIBCO);L15(美国GIBCO);FBS(美国ExCell Biology FBS500);MTT(美国Amresco);DMSO(溶解受试药品)(美国SIGMA D2650);土豆(沃尔玛);葡萄糖(源叶生物);琼脂粉(源叶生物);无水乙醇,分析纯(上海泰坦);次氯酸钠(分析纯,国药集团)。
-
人肝癌细胞HEPG2、人胃癌细胞HGC27、人结肠癌细胞HT-29、人宫颈癌细胞HELA(中国科学院上海细胞库)。
-
表面消毒:将采集新鲜济南、临沂和泰安产的戟叶牛皮消的根、茎和叶用自来水冲洗干净,转移至超净台,进行“75%乙醇-2.5%次氯酸钠-75%乙醇”的3步表面消毒处理。处理过后继续用无菌水冲洗5遍,灭菌滤纸将表面水分吸干。
组织块培养:超净台中操作,用消毒的剪刀和镊子分别将根、茎与叶剪切成小的组织块(0.5 cm×0.5 cm),分别从3个部位中各随机挑取20个组织块,每组设置4~5个组织块,分组好的组织块置于含有青霉素(50 mg/L)马铃薯葡萄糖琼脂培养基(PDA)的平板中,于温度25 ℃,湿度80%的恒温恒湿培养箱中进行内生真菌菌丝的生长情况的定期观察。挑取尖端菌丝转移到新的PDA培养基中培养,至菌丝形态单一,即得到纯化的菌株[15-16]。根据内生真菌菌株的培养的形态特征初步划分为不同的形态型,拍照留存。根据菌株群落的培养特征,划分为不同的形态型,继续将分离纯化后的菌株接种至PDA固体试管斜面培养基上进行培养,4 ℃冰箱保存。
-
观察培养的内生真菌菌落形态,对照《真菌鉴定手册》进行形态学特征鉴定。将“2.1.1”项下形态一致的泰山白首乌内生真菌菌株进行合并[16],参考E.Z.N.A.真菌DNA提取试剂盒说明书提取菌株DNA。以提取的DNA为模板,采用真菌ITS通用引物TIS4(5’-TCC TCC GCT TTA TTG ATA TGC-3’)和ITS5(5’-GGA AGT AAA GTC GTA ACA AGG-3’)对菌株的r DNA-ITS区域进行PCR扩增。PCR反应体系:2×Trans Taq Fidelity(HiFi) PCR SuperMix 15 μl,Primer(10 μmol/L)各1 μl,Genomic DNA 10 ng;补充双蒸水至 30 μl。反应条件:94 ℃预变性3 min,94 ℃变性40 s,52 ℃退火50 s,72 ℃延伸1 min,35个循环,72 ℃延伸10 min。5 μl PCR产物用2%琼脂糖凝胶电泳检测。将合格的PCR扩增产物送上海生工生物有限公司进行测序。内生真菌菌株测序得到的ITS序列去除载体序列,利用NCBI数据库(http://www.ncbi.nlm. nih.gov)BLAST进行比对,根据所得分子鉴定结果并结合形态学特征确定菌株。
-
按“2.1”项下方法分离得到的90个形态型泰山白首乌内生真菌菌株为供试菌株,PDA固体培养基中接种活化。待菌丝覆盖培养基表面时,用直径5 mm打孔器制备10个菌饼,放入装有100 ml的PDA培养基的锥形瓶中,每个菌种接种6瓶。接种后于25 °C、180 r/min 振荡培养7 d。发酵完成后,抽滤并收集发酵培养液,1∶1乙酸乙酯萃取3次,合并有机相,减压浓缩,即得乙酸乙酯提取物[17]。干燥后于4 °C冰箱中避光保存。
-
二甲基亚砜(DMSO)溶解乙酸乙酯粗提物后,用PBS分别稀释至0.001、0.01、0.1、1.0、10.0、100.0 μg/ml。采用MTT法测定样品抗肿瘤活性[17]。以人肝癌细胞HEPG2,人胃癌细胞HGC27,人结肠癌细胞HT-29,人宫颈癌细胞HELA为受试对象,阳性对照采用阿霉素。将细胞放置于含10% FBS、青霉素和链霉素各100 U/ml的DMEM细胞培养液中,于37 ℃、5% CO2饱和湿度的细胞培养箱中培养,48 h换液传代。消化传代后显微镜下观察细胞的生长情况。取对数生长期的细胞,胰酶消化后,10%小牛血清的完全培养液洗涤、悬浮,将100 μl悬浮细胞液(2~4×104个/ml)接种于96孔板中,培养24 h。吸弃培养液,每孔加入100 μl含有不同药物的完全培养基(含10%小牛血清,1%双抗),每种浓度设3个平行孔,设空白对照组,培养72 h后,每个孔加入5 mg/ml的 MTT 10 μl,培养4 h,吸弃培养液后加入100 μl DMSO,振荡至结晶完全溶解,用酶联免疫监测仪在波长为570 nm处测定A值,计算各浓度下的细胞抑制率,计算方法如下:
阴性对照孔相对A值=阴性对照孔绝对A值—空白对照孔绝对A值
药敏孔相对A值=药敏孔绝对A值—空白对照孔绝对A值
本研究采用SPSS 17.0通过机率单位加权回归法(Bliss法)计算IC50。
-
将分离到的869株内生真菌,根据培养特征划分为90个形态型,对不同形态型菌株ITS基因与GenBank中的参考序列进行分子系统学分析,结果见表1,有结果可知,鉴定得到的内生真菌属于3门、12纲、14目、14科、18属和30种。
属名 基因库中接近种(登录号) 相似度 (%) 组织部位 菌株数 根 茎 叶 链格孢属 A. alternata (MH368103.1) 99 1 2 3 A. alternata (MH716004.1) 99 4 4 A. alternata (MG669159.1) 99 1 1 A. alternata(MK07593.1) 99 1 1 A. alternata (MK392122.1) 99 1 1 A. alternata(MK659949.1) 99 2 2 A. alternata(KY859403.1) 99 2 2 A.alternata (KJ739880.1) 99 2 2 1 5 A. alternata (KY859403.1) 99 1 1 A. alternata (LN835252.1) 99 1 1 2 A. alternata (EF504974.1) 78 1 1 A. arborescens (MK460794.1) 99 1 1 A. brassicicola(MF167294.1) 99 1 1 2 A. burnsii(KR604840.1) 100 1 1 Alternaria sp.(KC139509.1) 99 1 1 Alternaria sp.(KC110624.1) 99 1 1 Alternaria sp.(KC147581.1) 99 2 2 Alternaria sp.(KU556507.1) 99 1 1 A.tenuissima(MG602685.1) 99 3 3 6 A. tenuissima (MK675103.1) 99 2 2 子囊菌属 Ascomycota sp.(FJ999646.1) 99 1 1 曲霉菌属 A. terreus var.floccosus (KP987086.1) 99 1 1 小檗属 B. fortunei (MK850215.1) 1 1 葡萄座腔菌属 B. dothidea(HM156069.1) 1 1 B. dothidea(KF294012.1) 1 1 双极霉属 B. sorokiniana (HF934936.1) 1 1 B. micropus(LT837454.1) 82 1 1 生赤壳属 B. ochroleuca(EU273558.1) 1 1 棒孢属 C.cassiiola (MH569606.1) 1 1 炭疽菌属 C. acutatum(MG661733.1) 1 1 C. capsici (EF016299.1) 1 1 C. gloeosporioides (KM044004.1) 1 1 C. nymphaeae (MH863840.1) 1 1 间座壳属 D. phaseolorum (MF379339.1) 1 1 D. phaseolorum (KX866874.1) 1 1 Emmia E. lacerate(MF101401.1) 1 1 突脐蠕孢属 E. rostratum (MH746929.1) 1 1 2 E. rostratum (MH746928.1) 1 1 镰刀菌属 F. nematophilum (KF577906.1) 2 2 F. nematophilum (KX621959.1) 1 1 F. oxysporum(MK673882.1) 1 1 F. oxysporum (KM005080.1) 1 1 F. oxysporum (KY910845.1) 1 1 F. oxysporum(GU724513.1) 1 1 F. solani f. batatas (AF178407.1) 6 7 F. solani batatas (EU625405.1) 1 1 F. solani batatas (MK571197.1) 1 1 F. solani f. batatas (KM235740.1) 1 1 F. solani f. batatas (KJ676962.1) 98 1 1 F. solani f. batatas (KU382502.1) 98 2 2 Fusarium sp.(FJ008989.1) 1 1 Fusarium sp. (MH884151.1) 1 1 小丛壳属 G. cingulata (EF423544.1) 2 2 球座菌属 G. mangiferae(EU677803.1) 1 1 孢菌属 Pleosporaceae sp. (HQ832799.1) 1 1 腔菌属 Pleosporales sp. (APBSDSF25) 1 1 P. cablin(MK568502.1) 98 1 1 毛球腔菌属 Setosphaeria sp. (LT837842.1) 92 1 1 踝节菌属 T. purpureogenus (KU981069.1) 1 1 炭角菌属 Xylariaceae sp. (MG669156.1) 1 1 28 30 32 90 -
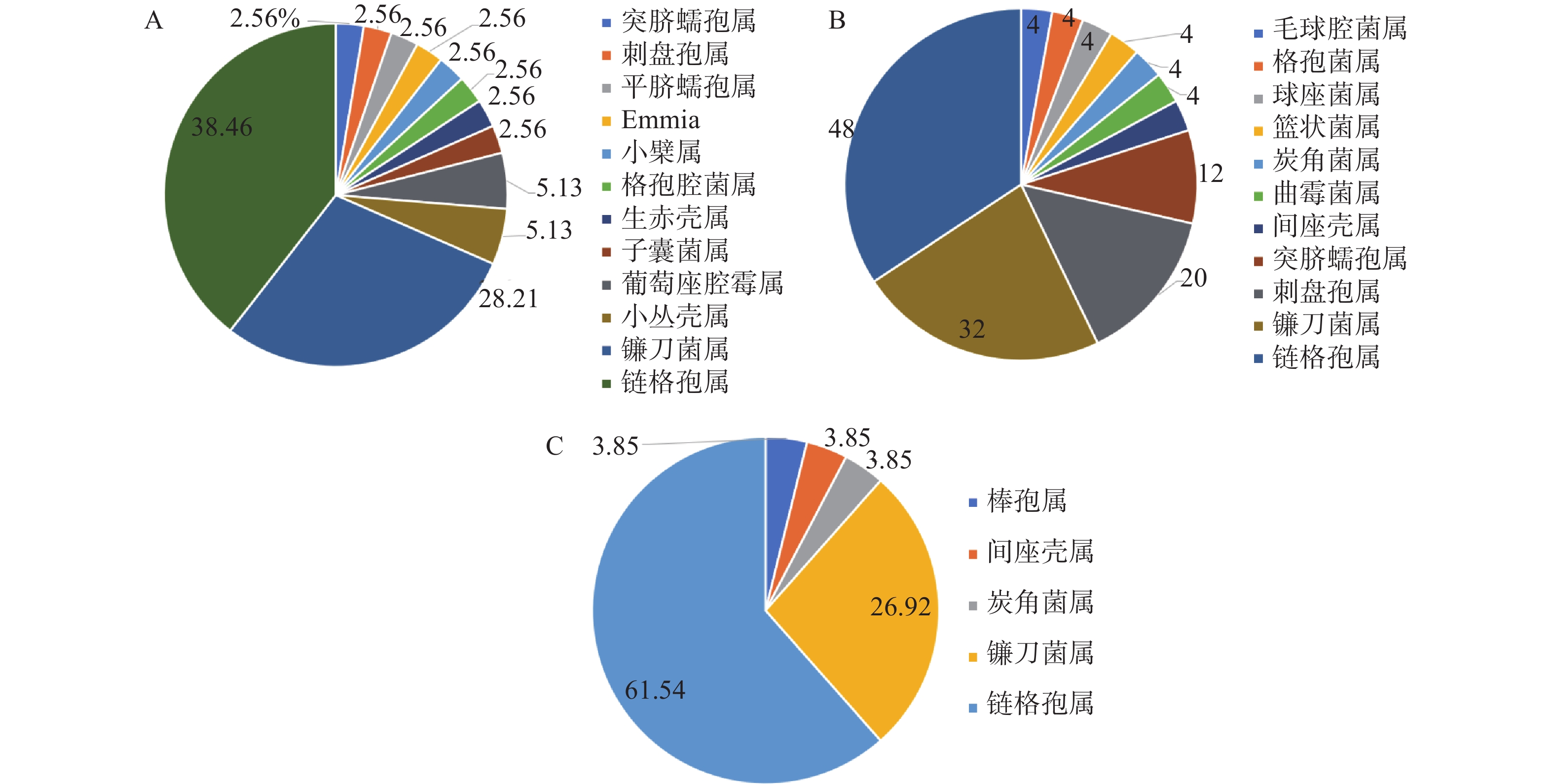
组织因素在影响内生真菌的多样性和分布规律发挥着重要的作用[18],在属的水平上,其对泰山白首乌内生真菌的组成影响也较为显著,如图1所示。泰山白首乌根部内生真菌主要分布于8个属,其中,优势菌属为镰刀菌属Fusarium,占根中内生真菌的64.29%;泰山白首乌茎部内生真菌分布于9个属,优势菌属为链格孢属Alternaria,占茎中内生真菌的60%;泰山白首乌叶部内生真菌分布13个属,优势菌属为链格孢属Alternaria,占叶中内生真菌的56.25%;泰山白首乌内生真菌的叶丰度大于茎和根。3个不同的组织部位中,链格孢属Alternaria和炭疽菌属Colletotrichum为三者共有属,其他具有差异。结果表明,在不同组织部位中,泰山白首乌的内生真菌的分布差异显著。
-
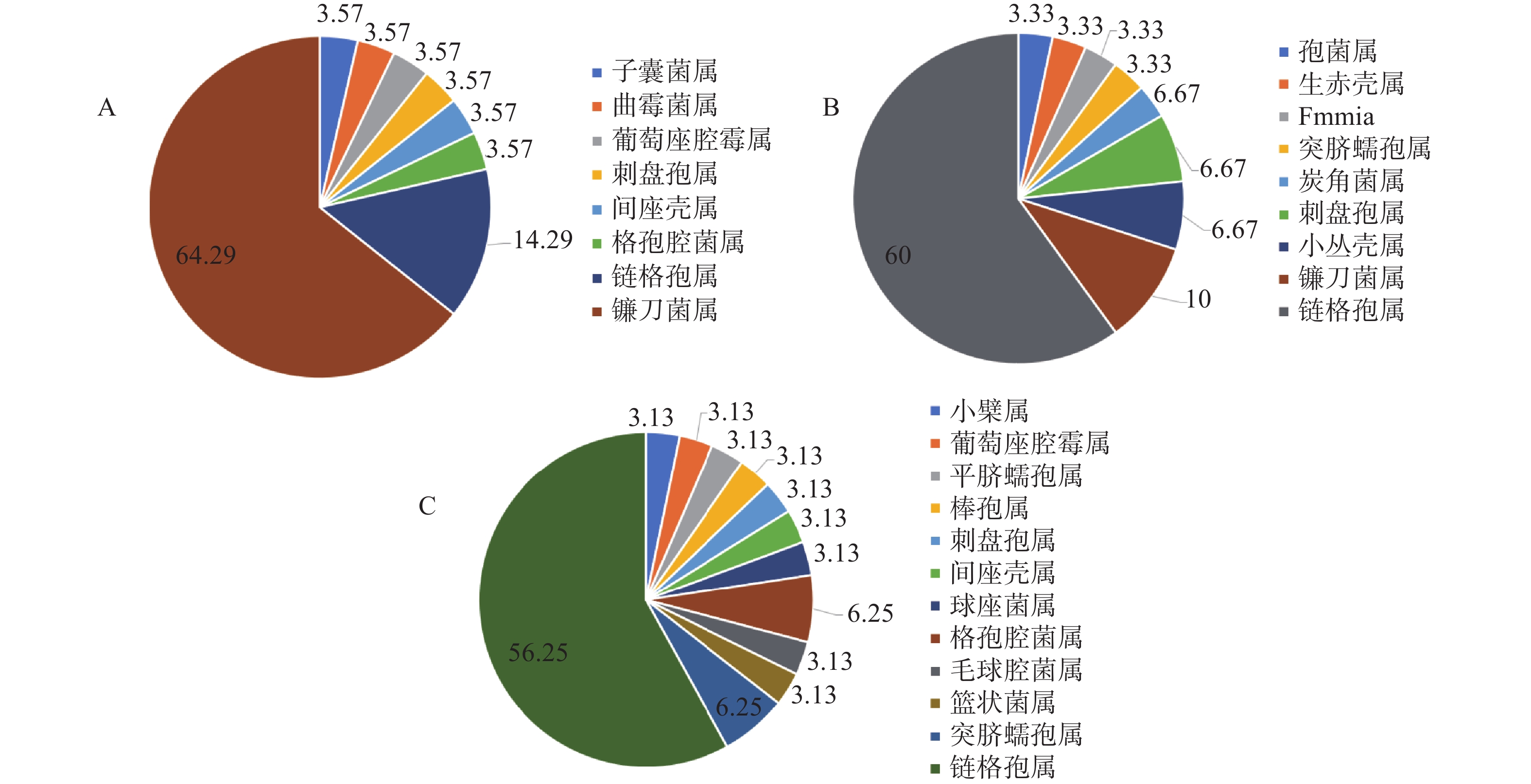
地理位置会影响内生真菌的多样性[19]。在90个形态型内生真菌菌株中,产地济南的泰山白首乌分离39个菌株,产地泰安分离得到26个菌株,产地临沂分离得到25个。如图2结果所示,3个产地的泰山白首乌优势菌属为链格孢属Alternaria和镰刀菌属Fusarium,产地济南的泰山白首乌内生真菌主要分布在12个属,优势菌属链格孢属占38.46%,镰刀菌属占28.21%;产地泰安的泰山白首乌内生真菌主要分布在5个属,优势菌属链格孢属占61.54%,镰刀菌属占26.92%;产地临沂的泰山白首乌内生真菌主要分布在11个属,优势菌属链格孢属占48.00%,镰刀菌属占32.00%。由此可知,产地对泰山白首乌内生真菌的群落组成和优势菌群均有影响,群落组成影响较大。
-
MTT结果表明,有13株内生真菌菌株代谢产物对HEPG2、HGC27、HT-29、HeLa肿瘤细胞株表现抗肿瘤活性,占总数的14.4%。如表2所示,B. sorokinianaJTY6、A. alternate JTY10、A. brassicicola JTJ11、B. ochroleuca JTJ18、Xylariaceae sp. LTJ1、A. tenuissima LTJ2、C. acutatum LTJ3和A. alternata LTJ6抗肿瘤活性较明显。链格孢属Alternaria是泰山白首乌内生真菌中筛选出抗肿瘤活性菌株的优势菌属,其中,A. tenuissima LTJ2和A. alternata LTJ6的抗肿瘤活性尤其显著,能够显著抑制HEPG2、HGC27、HT-29和HeLa肿瘤细胞株。A. tenuissima LTJ2对HEPG2、HGC27、HT-29、HeLa 4种肿瘤细胞株的 IC50值分别为(2.21±0.61)、(3.11±0.46)、(8.25±1.11)、(3.85±0.60) μg /ml;A. alternata LTJ6为(1.58±0.38)、(1.46±0.39)、(3.63±1.23)、(6.24±0.49) μg /ml。以上结果表明,A. tenuissima LTJ2和A. alternata LTJ6是泰山白首乌具有显著抗肿瘤活性的内生真菌株,可以进一步研究其产生抗肿瘤活性的单体成分。
菌株 抗肿瘤活性 (IC50, μg/ml) HGC27 HEPG2 HT-29 HELA JTY6 6.34±1.10 11.05±1.15 29.84±5.78 >40 JTY10 7.87±1.09 6.53±0.28 18.57±5.15 >40 JTJ11 9.92±1.13 6.59±0.56 5.94±0.88 21.37±5.99 JTJ18 2.61±0.35 3.20±0.42 3.55±0.30 9.96±2.38 LTJ1 1.69±0.32 2.96±0.24 13.23±1.66 7.41±1.47 LTJ2 2.21±0.61 3.11±0.46 8.25±1.11 3.85±0.60 LTJ3 5.34±0.89 5.10±1.21 13.01±1.63 5.87±1.36 LTJ6 1.58±0.38 1.46±0.39 3.63±1.23 6.24±0.49 JTJ13 21.76±0.68 >40 20.07±1.38 >40 LTJ5 21.43±0.35 33.43±1.31 >40 >40 LTJ10 21.34±0.65 29.81±0.32 >40 >40 TTY7 27.89±1.08 38.53±0.28 >40 >40 TTY18 18.25±0.24 >40 >40 31.41±1.49 阿霉素 0.022±0.003 0.034±0.01 0.030±0.003 0.039±0.006 -
泰山白首乌与“泰山黄精”、“泰山紫草”和“泰山四叶参”并称为泰山四大名药[2],但因其自然繁殖率低等因素,导致资源匮乏,市场上供不应求。植物内生真菌与宿主长期协同进化,可以产生相同或相似的活性代谢产物[9],通过对泰山白首乌内生真菌的深入研究将有效的缓解其资源匮乏,而内生真菌有可能成为开发泰山白首乌的新资源。
本研究表明泰山白首乌中内生真菌资源丰富,具有较丰富的多样性,泰山白首乌内生真菌的分布在不同组织部位差异显著,以丰度比较叶大于茎和根,具有明显的组织特异性,产地对泰山白首乌的优势菌群和群落组成有影响。除优势属、种外,分离得到的大豆疫霉、炭角菌和淡色赤壳菌等内生真菌菌株,也具有良好生物活性 [20-22]。
植物内生真菌能产生与宿主相同或相似的活性成分及生物活性,本研究首次报道了筛选得到的13株泰山白首乌内生真菌具有抗肿瘤活性,占总数的14.4%,其中,从叶中筛选到4株抗肿瘤活性菌株,茎中筛选得到9株抗肿瘤活性菌株,根中无,同时,A. tenuissima LTJ2和A. alternata LTJ6两种抗肿瘤活性尤其显著,值得深入研究。然而,泰山白首乌的药用部位为块根,由于内生真菌在植物组织中的定殖不同,导致在不同组织部位的分布存在差异,具体原因还需要进一步深入研究。另外,从产地上来看,不同产地所筛选得到的抗肿瘤活性菌株数量及品种不同,济南产泰山白首乌筛选到5株抗肿瘤活性菌株,临沂产泰山白首乌筛选到6株活性菌株,泰安产泰山白首乌筛选到2株活性菌株,综上,泰山白首乌中内生真菌的种群结构的抗肿瘤活性是否存在与产地相关,需要进一步深入研究,而已经分离得到的活性菌株的次生代谢产物及其作用机制的研究也是下一个重要目标。
Anti-tumor activities of endophytes from Cynanchum bungei Decne.
doi: 10.12206/j.issn.1006-0111.202108089
- Received Date: 2021-08-18
- Rev Recd Date: 2021-10-25
- Available Online: 2021-12-27
- Publish Date: 2021-11-25
-
Key words:
- C. bungei /
- Alternaria /
- anti-tumor activity
Abstract:
| Citation: | FENG Kunmiao, WU Sijia, CHEN Wenhua, DAI Wei, XU Lingchuan, HAN Ting. Anti-tumor activities of endophytes from Cynanchum bungei Decne.[J]. Journal of Pharmaceutical Practice and Service, 2021, 39(6): 542-548. doi: 10.12206/j.issn.1006-0111.202108089 |







 DownLoad:
DownLoad: