-
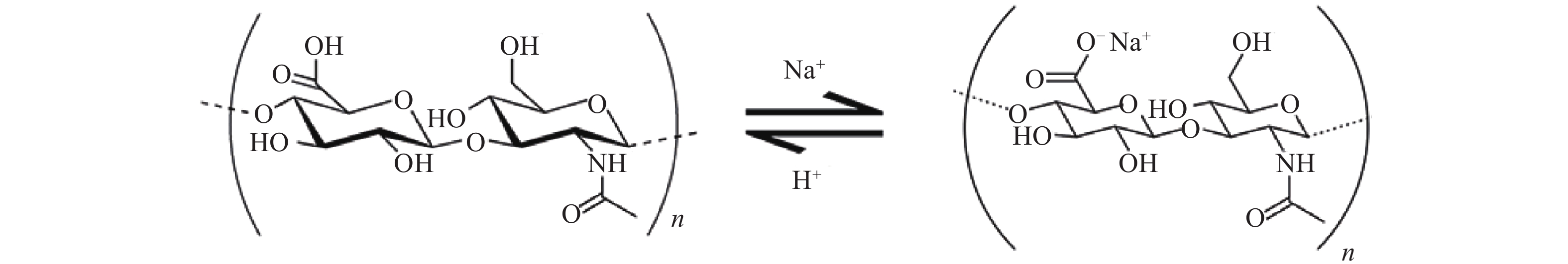
透明质酸(hyaluronic acid, HA)又名玻璃酸。首次在1934年由Meyer和Palmer自牛眼玻璃体内提取分离[1]。HA同样广泛存在于人体中,包括人眼的玻璃体、脐带和皮肤等组织(表1),是细胞外基质的组成部分[2]。HA化学名称为(1,4)-O-β-D葡萄糖醛酸-(1,3)-2-乙酰氨基-2-脱氧-β-D葡萄糖,是一种高分子直链聚糖,由交替的N-乙酰葡糖胺(GlcNAc)和葡糖醛酸(GlcA)双糖单位反复交替而形成的一种聚合物[3],其分子量的差别很大,分子式为(C14H20NO11Na)n,双糖单位的分子量为401.3(图1)。由于其直链的链轴L单糖之间氢键的作用,HA分子在空间呈刚性的螺旋柱型,其半径为200 nm。HA呈强亲水性,在水溶液中,HA亲和的水分约为其本身重量的1000倍。除了亲水性外,HA溶液还有着独特的流体力学性质,其水溶液是一种非牛顿型流体,因此,有着良好的黏弹性和应变性[4]。目前HA广泛应用于生物材料、药物靶向制剂、美容以及腹部手术后预防粘连等[5]。
组织或体液 HA浓度(μg/ml) 眼玻璃体 140~340 脐带 4100 关节滑液 1400~3600 真皮 200~500 表皮 100 胸淋巴液 0.2~50 尿液 0.1~0.3 血清 0.01~0.1 -
HA作为一种酸性黏多糖,广泛分布于人体各种组织的细胞间质中,具有维持细胞渗透压稳定,使相邻细胞黏合等重要生理功能[6]。另外,HA还能调节细胞的黏附和运动功能,调控细胞分化和增殖,维持组织的生物力学性能正常[7]。HA在眼科手术中具有广泛应用,主要是由于其具有黏性、假塑性、弹性、黏合性和涂布性等特性,这些特性使其具有黏弹性衬垫、组织内分离、黏性阻塞、黏性止血、黏弹性缓冲以及弹性固定等重要功能[8]。
干眼病是一种常见的眼科综合症,主要由眼腺细胞功能紊乱等原因所致,包括各类结膜炎等。HA富含亲水基团,能够与水分子结合,起亲水及润滑作用,因此可以在一定程度上缓解干眼症状。《干眼临床诊疗专家共识(2013)》指出[9],“干眼症治疗目的:轻度干眼症患者是缓解眼部症状,严重干眼患者是保护视功能”。透明质酸钠滴眼液可以冲洗并稀释眼表面炎性介质,降低泪液渗透压,促进眼表上皮的愈合,以及促进眼表纤维连结蛋白分泌和沉积,从而在眼表上皮损伤愈合中起重要作用。俞惠玲[10]对106例干眼症患者的研究发现,单独使用0.3%透明质酸钠滴眼液,对干眼症有效率达81.8%;透明质酸钠滴眼液加普拉洛芬滴眼液联合使用有效率高达92.3%。
-
HA常用于化妆品保湿成分,包括HA保湿锁水霜以及保湿补水面膜等。人体内50%HA存在于皮肤真皮内,为胶原纤维和弹性蛋白的分布提供空间架构。三者共同形成皮肤的支架,维持皮肤组织稳定,保持皮肤弹性。若有一者缺失,可加速皮肤衰老和皱纹的形成[11]。HA作为组成人体结缔组织和滑液的成分之一,因其生物相容性较高,是目前全球用量最大的皮肤填充物之一,常用于眼周眉间纹、鱼尾纹、抬头纹等治疗[12-14]。薛紫涵等人通过对23例轻中度面部松弛患者,进行面部韧带处注射HA 0.2 ml,23例患者未出现红肿、疼痛、瘀青和过敏反应,也未发生硬块结节、填充物移位、血管栓塞、皮肤坏死等不良反应。注射后,所有人均有不同程度的眼角和颧下松弛的改善,并有提升法令纹、口角上扬、下颌缘更清晰以及面部更加紧致等效果。随访表明,注射效果约持续3~6个月,若欲维持效果则需半年后再次注射[15]。HA能够调控胶原的合成,降低炎性介质的生成,抑制创面毛细血管的渗出和纤维蛋白原的沉积,抑制成纤维细胞合成胶原纤维,从而抑制手术瘢痕形成[16-18]。Patel采用无针注射交联HA,治疗了两例痤疮疤痕患者,间隔4周后,再重复给药一次,能有效减轻病人的疤痕程度,无不良反应[19]。
-
HA是关节滑液的重要成分,作为润滑剂给骨骼末端提供保护[20]。在最初合成时,HA主要由2×105~2×106 Da之间的高分子量HA聚合物组成[21]。在骨关节炎和类风湿性关节炎,HA分子量变小,滑液黏稠度降低,使得HA黏弹性下降,从而导致关节面的磨损增加[20]。关节腔内注射高分子量HA能有效的缓解骨关节炎和类风湿性关节炎患者的疼痛,起到一定的治疗作用[22]。HA除了润滑作用外,还能降低巨噬细胞的吞噬作用,改善炎症反应[23]。苑树岩等给35例膝关节炎患者的关节腔内注射2.5 ml 透明质酸钠,给药后患者疼痛减轻,关节滑液中IL-6β含量下降[24]。Seung发现交联HA凝胶支架易与人牙髓干细胞和模拟肽混合,混合溶液便于注射。注射给小鼠后,交联HA迅速在注射部位形成水凝胶支架,并可安全无刺激的在小鼠体内保留8周以上。牙髓干细胞在水凝胶中至少可存活8周,模拟肽可诱导牙髓干细胞向成骨细胞分化,交联HA凝胶支架作为生物材料,可以用于骨骼组织工程[25]。
-
HA用于外科手术后防止组织黏连,能有效的降低黏连的发生率和减轻黏连的严重程度[26]。HA防止术后组织黏连的机理是:①HA凝胶具有高分子纤维网状结构,涂布于组织表面,能起到阻隔作用,在腹膜修复时,形成一种短暂的保护屏障;②抑制术后出血和渗出,减少能形成永久性黏连骨架的血块数量,避免组织接触面的纤维蛋白沉着;③HA抑制中性粒白细胞迁移和吞噬作用,降低炎性反应[27];④HA与间质细胞和成纤维细胞膜表面高亲和力的HA受体蛋白相互作用,提高这些细胞的迁移及趋化能力,从而促进了体内修复过程;⑤HA凝胶覆盖于创伤浆膜表面,在一定时间内不被降解代谢,使早期的创面组织修复能持续有效的进行,直至在创面形成一连续的间皮细胞覆盖层,完成组织修复。
蔡同凯等[28]采用大鼠盲肠和腹壁双侧损伤模型,以Nair黏连5级分类法判断腹腔黏连程度,发现术后30 d,透明质酸钠凝胶组SD大鼠腹腔黏连明显低于模型组,透明质酸钠凝胶组极限荷载比和刚度比与假手术组和模型组无统计学差异。医用透明质酸钠凝胶能有效降低黏连程度,并且不影响创面的恢复。Zhang等采用大鼠两次子宫损伤模型,研究了医用透明质酸钠和氧化再生纤维素预防大鼠腹膜黏连的疗效,发现医用透明质酸钠和氧化再生纤维素均能有效的降低黏连损伤的程度[27]。固体医用膜在使用时,难以被准确固定在创伤部位,在组织愈合后,还需要再次手术取出医用膜[29]。液体形式的HA,不仅便于操作,易于覆盖在创伤部位,而且其在体内保留的时间与伤口愈合周期一致。
-
近年来,HA作为药物载体,与其他药物反应形成化合物,发挥缓释作用和靶向作用,使其结合的药物能够定时或定向地被释放[30-32]。HA作为纳米材料,可以与抗肿瘤药物形成靶向制剂,治疗盆腔肿瘤。Lee发现透明质酸纳米粒易于被CD44受体阳性患者的结肠癌HCT116细胞内吞吸收[33],HA与紫杉醇共轭形成的纳米复合物,体外对HCT116细胞显示出更强的细胞毒作用[34]。Bajaj将紫杉醇用HA胶体包裹,延长了紫杉醇在裸鼠体内的存留时间,并有效减少人卵巢癌SKOV-3肿瘤的生长和转移[35]。Xiao等采用透明质酸-十八烷基胺结构的脂质载体,装载紫杉醇,其装载率被提高到72%。在裸鼠体内,装载紫杉醇的透明质酸-十八烷基胺载体在肝脏和脾脏的分布减少,而在肿瘤组织的分布增加[36]。Choi将伊立替康封装在HA纳米粒中,在裸鼠体内能有效抑制人结肠癌CT29肿瘤的生长,同时能降低伊立替康的不良反应。利用荧光技术可以观察结肠癌CT26肿瘤的转移[37]。Zhang等利用环丙沙星和万古霉素与HA偶联制备为抗生素缓释颗粒,能在一周内有效抑制绿脓杆菌、金黄色葡萄球菌和枯草芽孢杆菌[38]。
-
人体内HA主要由HA分解酶(Hyals)分解,其中最主要的酶是Hyal-1和Hyal-2。Hyal-2将HA降解成低分子量HA,Hyal-1将HA降解成低分子寡聚体。高分子量HA仅β1、4键暴露,降解缓慢,当HA分子量低于30万时,HA聚集能力降低,降解速度呈指数倍增加[39]。有报道[40]HA能被降解为多糖,为葡萄球菌和链球菌等提供营养。Zhang等[41]研究表明,在富含HA的培养基中,化脓性链球菌的M1蛋白、胶原样表面蛋白和糖酵解酶甘油醛-3-磷酸脱氢酶等几种致病因子上调。但是也有报道[42-43],HA能抑制SA等细菌的生长,1 mg/ml浓度时就能达到最大抑制作用,但是没有杀菌作用。高浓度高分子量HA体外抑制SA和大肠埃希菌的生长,并且体内体外均不影响抗生素的药效[28]。Jae采用小鼠盲肠结扎穿刺法,构建小鼠腹腔脓肿模型,通过腹腔注射高分子量HA(20 mg/kg),能有效的降低腹腔菌载量,降低炎症因子水平,提高小鼠的存活率[44]。
-
内源性低分子量HA可以促进血管生成,增加肿瘤细胞的供血,促进肿瘤细胞的生长[45-47];另外,内源性低分子量HA也可以促进肿瘤细胞表面CD44的分泌,从而促进肿瘤细胞的转移[48]。通过抑制透明质酸合成酶3(HAS3),降低低分子量HA的产生,可使前列腺肿瘤血管生成减少70%~80%,肿瘤生长速度降低[49]。透明质酸合成酶2(HAS2)过表达,高分子量HA合成增加,组织中高分子量HA浓度升高,反而体现出抑制肿瘤细胞的生长[50]。也有文献报道,内源性的HA能促进肿瘤细胞增殖和扩散[51-53]。外源性HA根据分子量不同,应用也不同[32,54](表2)。外源性高分子量HA进入人体后会促进HAS2高表达,加速内源性高分子量HA合成,使机体高分子量HA浓度增加,从而抑制肿瘤的生长和扩散[55]。Aikaterini用外源性高分子量HA显著抑制 HT1080细胞的迁移,当加入HA分解酶水解高分子量HA后,HT1080 细胞运动性显著增加[56]。高分子量HA钠凝胶能够抑制结肠癌细胞的转移[57]。除了和抗肿瘤药物合用,外源性寡聚体HA单用还可以与CD44 受体结合,增强肿瘤细胞的凋亡[58],与HA介导的运动受体结合,降低肿瘤细胞的转移[59]。
用途 分子量(Da) 作用 骨关节注射液 >5×106 对受损部位润滑和机械保护作用; 眼科手术黏弹剂 >3×106 撑起前房,减少术后炎症的发生 滴眼液 (1~1.5)×106 增加滞留时间;润滑和保护作用 术后防黏连 >1×106 形成隔离层,抑制炎症反应 化妆品 (0.5~2)×106 保湿 抗肿瘤药载体 (5~7.5)×105 与肿瘤CD44靶点特异性结合 对创伤修复 <5×105 促进巨噬细胞的吞噬作用;促进血管形成 抗肿瘤作用 800~3200 特异性结合CD44受体和RHAMM受体 -
HA的结构与功能决定了其可用于生物材料、药物靶向制剂、美容以及腹部手术后预防黏连等,但是随着HA可能促进肿瘤细胞的生长和转移以及促进细菌的生长等疑问的出现,使得临床使用HA变得慎之又慎。
本文对HA的临床应用和HA对肿瘤以及细菌作用机制进行综述,对临床安全使用HA特别是将HA用于腹盆腔肿瘤患者的术后防黏连具有一定的指导意义,但其相关作用机制还需要进一步研究。
Research progress on action mechanism and clinical application of hyaluronic acid
doi: 10.12206/j.issn.1006-0111.202108002
- Received Date: 2021-08-02
- Rev Recd Date: 2021-10-11
- Available Online: 2022-05-25
- Publish Date: 2022-03-25
-
Key words:
- hyaluronic acid /
- biomaterials /
- drug targeted preparations /
- cosmetology /
- prevent postoperative adhesion
Abstract: Hyaluronic acid is widely present in the human body. It is an important component of extracellular matrix. It has unique hydrodynamic properties, good viscoelasticity and strain properties. At present, hyaluronic acid has been widely used in biomaterials, targeted-drug preparations, cosmetics and prevention of adhesion after abdominal surgery. With the expansion of the application scope of hyaluronic acid and the continuous emergence of new medical materials, the research on hyaluronic acid has been increasing in recent years. This paper reviews the clinical application of hyaluronic acid and its mechanism, in order to provide reference for the further development and safe application of hyaluronic acid products.
| Citation: | CAI Tongkai, LIU Mouzhi, DENG Jie, CAO Yongbing, YAN Lan. Research progress on action mechanism and clinical application of hyaluronic acid[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(2): 103-107, 131. doi: 10.12206/j.issn.1006-0111.202108002 |








 DownLoad:
DownLoad: