-
细胞间隙连接(Intercellular gap junction, GJIC)是一种存在于人体所有细胞中的膜通道,由连接蛋白(connexins, Cxs)形成,并负责转移生物活性分子、代谢物和相邻细胞或细胞与细胞外环境间的盐离子,对细胞的增殖、分化及机体内环境稳定、新陈代谢、生长发育起至关重要作用[1]。实验证实小鼠骨髓、肝脏及脾脏基质中有11种不同的连接蛋白表达,但人类骨髓基质中仅仅有3种Cxs (Cx31、Cx43、Cx45)表达。多项实验均证实Cx43在支持正常造血过程中具有重要作用,而我们前期的研究发现,Cx43在多发性骨髓瘤(multiple myeloma,MM)的发病过程中具有重要作用,患者骨髓微环境中的Cx43表达水平较正常明显升高,骨髓瘤细胞与成骨细胞相互作用后可通过由Cx43组成的GJIC促进其迁移,上调Cx43表达对多发性骨髓瘤细胞的增殖及迁移均起到促进作用,Cx43表达异常与骨髓瘤融合细胞发生相关[2-3]。然而Cx43在MM细胞生存及耐药中的作用尚未阐明,尤其在多发性骨髓瘤干细胞及其与微环境中作用尚不明确。有鉴于此,本研究分离、培养MM患者及正常志愿者来源骨髓间充质干细胞(MM-MSCs、ND-MSCs),在直接共培养条件下观察MM干细胞样细胞生物学特性的变化及MM-MSCs对MM干细胞样细胞的生存及耐药的作用,并探讨其可能机制。
-
细胞株:MM细胞株RPMI 8226、U266、XG4、XG7(苏州大学生物技术研究所张学光教授惠赠);试剂:FBS、PBS、LG-DMEM完全培养液、RPMI1640培养基(美国Gibco公司);Midi MACs系统(德国Milteyni公司);Hoechst33342(美国Sigma公司);抗Cx43及GAPDH一抗(美国CST公司);RNeasy kit试剂盒、QuantiTect reverse transcriptase kit试剂盒、TopTaq Master Mix Kit 试剂盒(美国Qiagen公司);Cytometric Beads Array试剂盒(美国BD公司)。
-
参考文献[4]的方法,采用Ficoll分离MM患者及正常志愿者骨髓单个核细胞(BM-MNCs),用含10%FBS的LG-DMEM完全培养液培养,观察细胞状态,72 h后首次换液,以后根据情况每2~3 d,换液1次。待细胞生长至80%融合后,胰酶消化传代。传至第三代后收获细胞进行后续实验,剩余细胞标记后冻存于液氮罐中备用。志愿者及患者骨髓间充质干细胞(BM-MSCs)的分离、扩增和鉴定在知情同意下获得,并经医院伦理委员会批准。
-
RPMI 8226、U266采用含有10% FBS的RPMI1640培养基培养。XG4、XG7采用含有10% FBS、1 ng/ml IL-6的RPMI1640培养基培养。原代MM细胞来自6例初诊MM患者骨髓:用Ficoll分离BM-MNCs,并用Midi MACs系统纯化,留取CD38+、CD138+细胞,操作按说明书进行,分选后的细胞采用流式细胞术(FCM)检测其纯度,CD38+、CD138+细胞≥90%,采用含有10% FBS的RPMI1640培养基培养。观察细胞状态,48 h后首次换液,以后根据情况每1~2 d,换液1次。传至第三代后收获细胞进行后续实验,剩余细胞标记后冻存于液氮罐中备用。
-
取对数生长期F3代MM-MSCs及ND-MSCs,用PBS洗涤后,调整细胞浓度为2.0×106/ml,每取100 μl细胞悬液,分别加入PE标记的CD90、CD73、CD44、CD105、CD34、CD45及HLA-DR,阴性对照为PE标记的同型IgG,室温下孵育30 min,PBS洗涤2次后,FCM上机检测。
-
按文献[5]报道的方法,分别取RPMI 8226、U266、XG4、XG7及原代MM细胞,调整细胞浓度为106/ml,加入浓度为1 mg/ml的Hoechst33342,调整其终浓度为5 μg/ml,混匀后置于37 ℃水浴箱中避光孵育120 min,期间数次晃动离心管。对照组于此步骤中加入终浓度为50 μmol/L维拉帕米同时孵育。离心后PBS洗涤,用含碘化丙啶(2 μg/m1)的4 ℃预冷PBS重悬细胞,并置于冰浴中。FCM上机检测,激发波长为350 nm,采集波长为450 nm(蓝光)和675 nm(红光),通过与对照组比较,选取染色偏弱部分的细胞即为SP细胞。SP细胞分选按上述步骤准备细胞,ALTRA流式细胞仪更换鞘液并用酒精进行清洗后换为双蒸水冲洗;分别上Hoechst33342管和Hoechst33342+verapamil管进行检测,FCM选择SP分选方案,调整分选参数,全程需要振荡,分选结束后在无菌条件下分别收集主群细胞(MP)和侧群细胞(SP),备用。
-
分别收集RPMI8226、SP细胞、ND-MSCs、MM-MSCs、SP细胞+ND-MSCs、SP细胞+ND-MSCs+25 mmol/L α-GA、SP细胞+MM-MSCs和SP细胞+MM-MSCs+25 mmol/L α-GA各组细胞,用预冷的PBS洗涤细胞3次,加入细胞裂解液,置4 ℃作用30 min,12000 g/min离心10 min,收集上清液,BCA法测定蛋白浓度,加入4×SDS凝胶加样缓冲液混匀,煮沸10 min使蛋白变性。然后,行聚丙烯酰胺凝胶电泳(SDS-PAGE),并转移至PVDF膜上,封闭1 h后,分别与抗Cx43及GAPDH一抗4 ℃孵育过夜,TBS液洗涤后再与HRP标记的二抗共孵育1 h,洗涤后,应用ECL化学发光法显象和Image图象分析软件分析。
-
采用碘化吡啶(PI)法。实验分组:①SP细胞+MM-MSCs;②SP细胞+MM-MSCs+25 mmol/L 18α甘草次酸(α-GA);③对照组为RPMI 8226细胞。实验设3复孔,FCM分析其DNA含量,CellQuest软件分析结果。
-
采用甲基纤维素半固体培养法。实验分组:①SP细胞+MM-MSC组;②SP细胞+MM-MSC+25 mmol/L α-GA组。分别调整SP细胞和MM-MSC细胞浓度为4×105/ml和2.0×106/ml,与等量的2%甲基纤维素混均后,接种于6孔板,每孔总体系2 ml;置饱和湿度、37 ℃的CO2培养箱中培养,14 d取出,置倒置显微镜下记录集落数,≥50细胞为集落,≤50则为簇。
-
采用逆转录聚合酶链式反应(RT-PCR)方法。实验分组:①RPMI 8226组;②新鲜分离SP细胞组;③SP细胞+MM-MSC组;④SP细胞+MM-MSC+25 mmol/L α-GA组。收集各组细胞,操作按试剂盒说明进行。简述如下:采用RNeasy kit试剂盒提取RNA样本,取1μg RNA进行逆转录,按等量cDNA进行PCR反应。所有引物序列均由上海生物工程公司设计并合成,采用β-actin为内参。β-actin上游引物 5′-TCCTGTGGCATCCACG AAACT-3′,下游引物 5′-GAAGCATTTGC GGTGGACGAT-3′,其它引物见表1。PCR扩增条件均为:94 ℃ 5 min、94 ℃ 40 s、56 ℃ 30 s、72 ℃ 32 s,共35个循环。取4 μl PCR产物、Marker 3.5 μl分别加样于2.0%琼脂糖凝胶中电泳,电压100V电泳30~60 min,紫外投射仪观察目标条带,摄影,图象分析软件Smartview2001分析处理结果。
基因 引物序列 c-myc 5′CTTCTCTCCGTCCTCGGATTCT
3′GAAGGTGATCCAGACTCTGACCTTKlf-4 5′GCAAGTCCCCTCTCTCCATTA
3′GTAAGGTTTCTCGCCTGTGTGOct-4 5′GGAGATATGCAAAGCAGAAACC
3′CTCAAAATCCTCTCGTTGTGCSox-2 5′CGGCAACCAGAAAAACAGC
3′TCTCCGTCTCCGACAAAAGT -
采用CBA检测法。取对数生长期MM-MSCs,调整细胞数1×105/ml 接种于6孔板,培养箱静置4 h弃上清,并将不同MM细胞按1×105/ml的浓度接种该孔中,每孔2 ml,分组为:①RPMI 8226细胞;②SP细胞+MM-MSCs;③SP+MM-MSCs+α-GA(25mmol/L);④MM-MSCs。每组设3个复孔,培养24 h后收集培养上清,利用CBA技术测定上清中IL-6、IL-10、TGFβ、bFGF和IL-17的变化。
-
采用annexinV/PI标记细胞流式术分析法。取对数生长期MM-MSC细胞,调整细胞数4×105/孔接种于24孔板;RPMI8226或SP细胞,调整细胞数2×104/孔接种于24孔板,培养箱静置4 h后去上清,分组如下:①RPMI8226;②RPMI8226+硼替佐米(BTZ);③SP+BTZ;④SP+MM-MSC+BTZ;⑤SP+MM-MSC+BTZ+α-GA。所有实验组BTZ及α-GA的终浓度分别为20 nmol/L和25 mmol/L,培养24 h后收集细胞,FCM检测细胞凋亡,实验设5复孔。
-
所有数据采用Graphpad Prism 5.0 统计处理软件分析,以均数±标准差表示。组间分析采用t检验,P<0.05 为差别具有统计学意义。
-
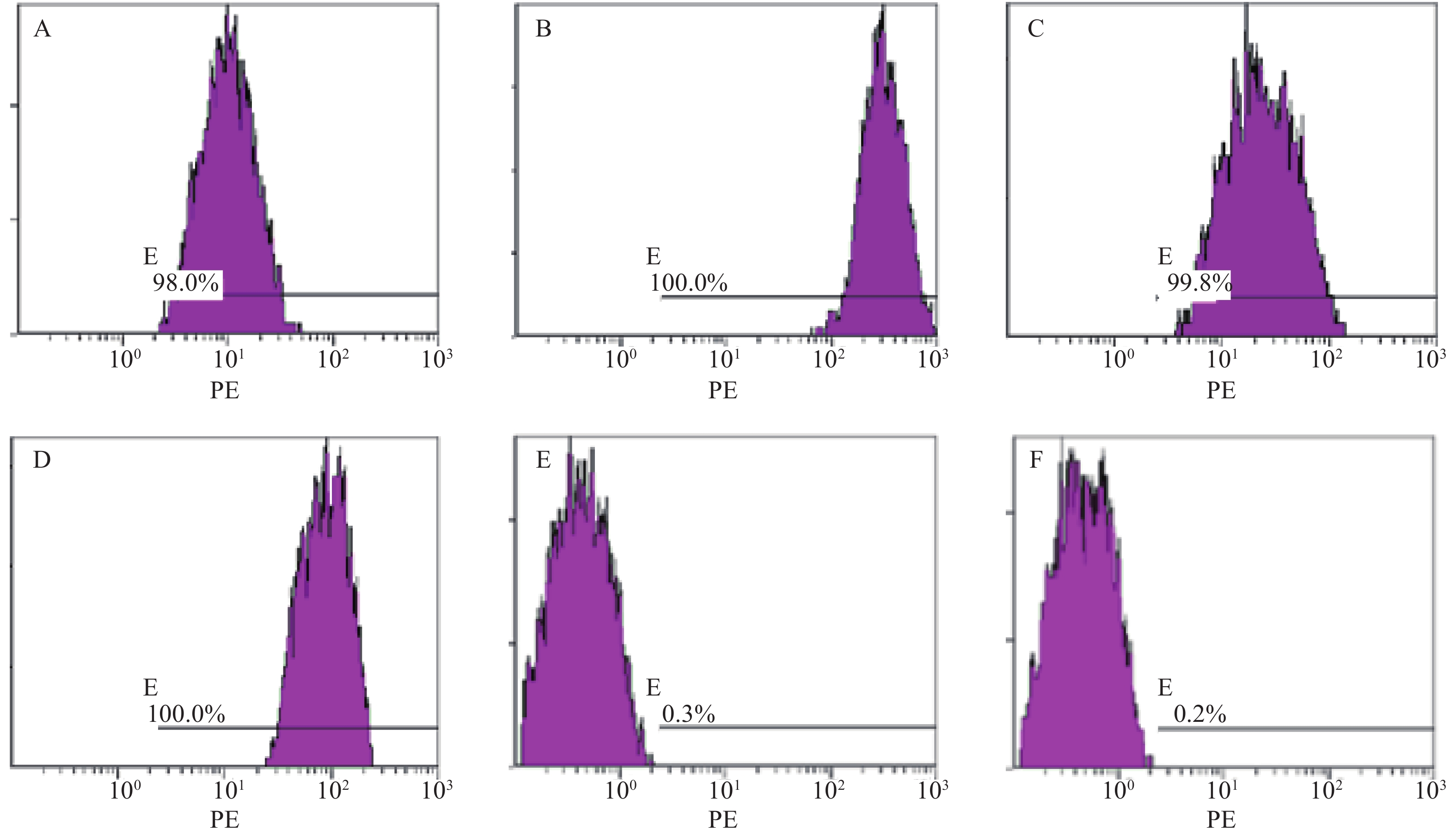
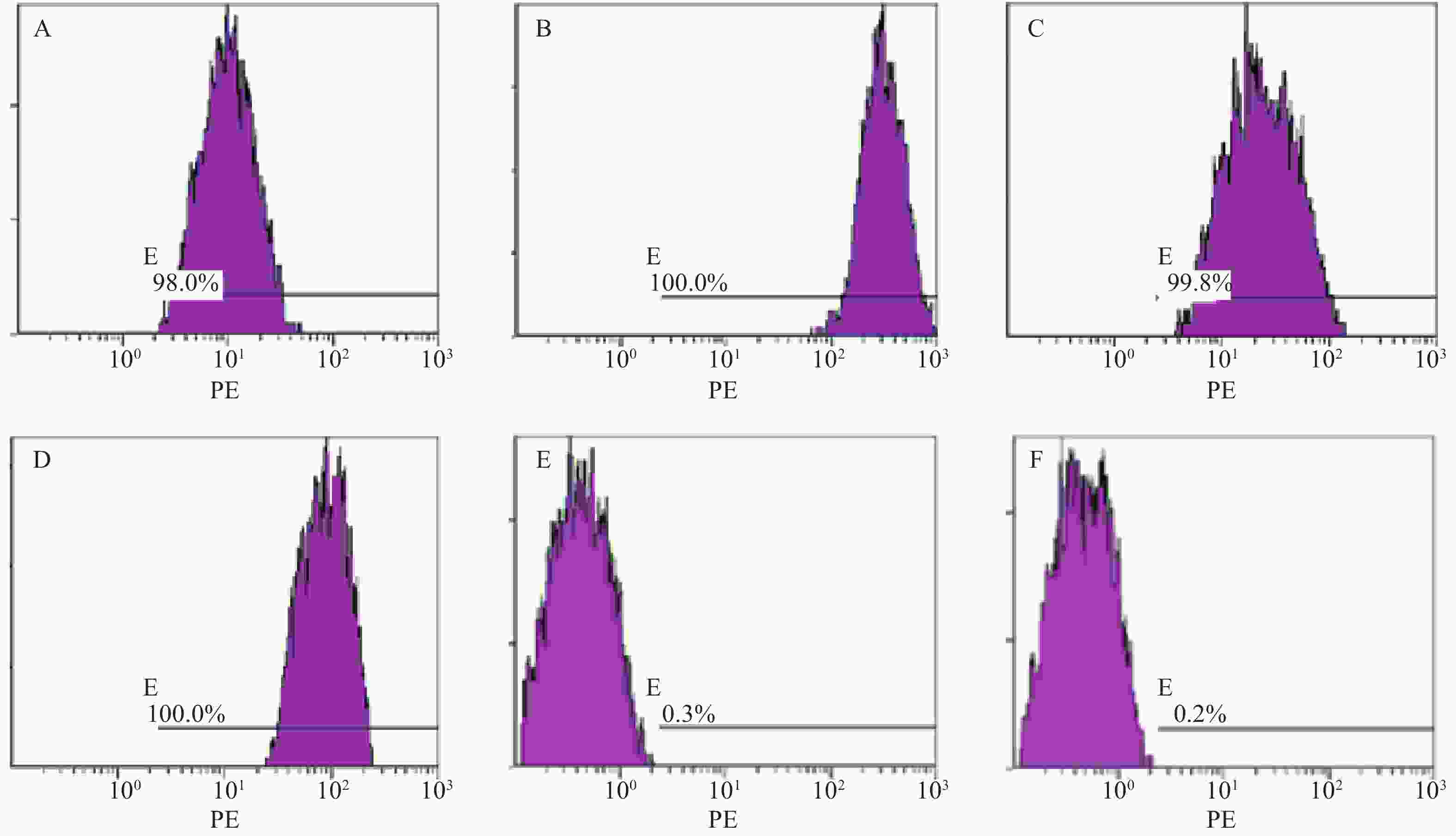
分离培养获得MM-MSCs及ND-MSCs,表面抗原提示两者均为高表达CD73(98.0%)、CD44(100%)、CD90(99.8%)和CD105(100%),基本不表达CD34(0.3%)、HLA-DR(0.2%),细胞形态两者无明显差异。见表2和图1。
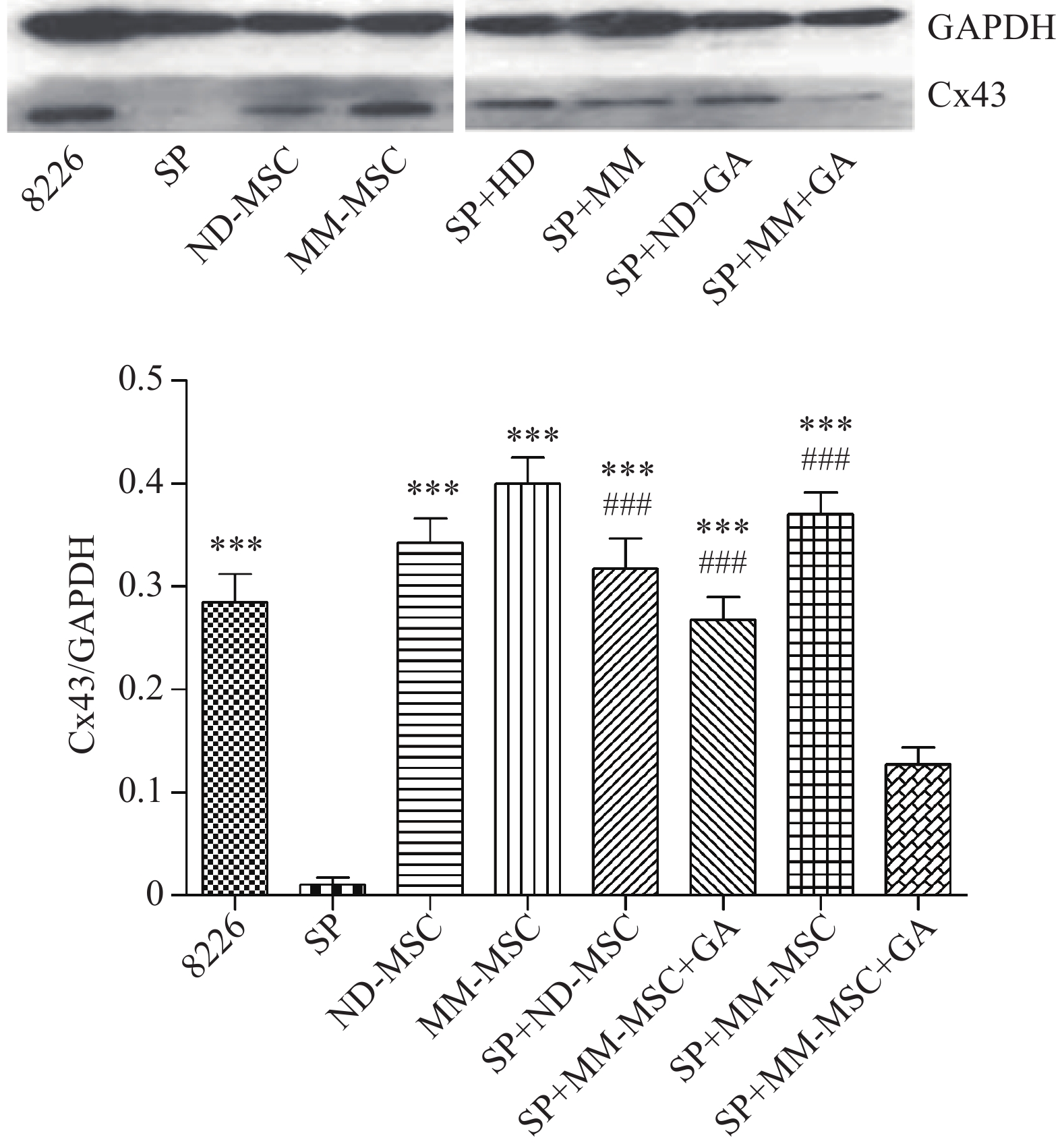
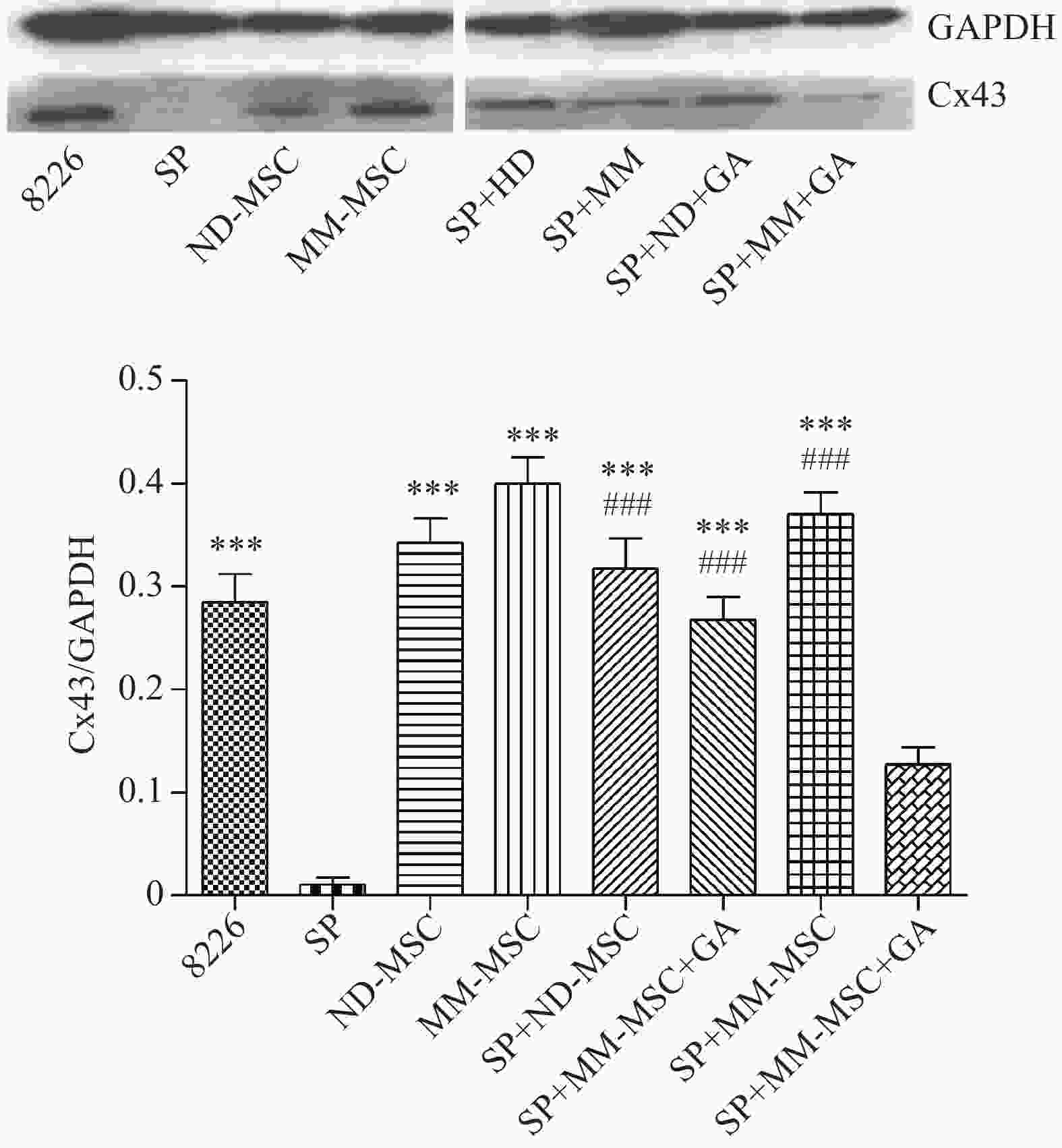
表面抗原 CD73 CD44 CD90 CD105 CD34 HLA-DR 表达率(%) 98.00 100 99.80 100 0.30 0.20 蛋白印迹试验证实SP细胞仅表达极少量的Cx43分子,而RPMI 8226细胞则表达较高水平的Cx43,两者具有显著性差异(P<0.001);MM-MSCs较ND-MSCs表达Cx43明显较多,但不具有统计学意义(P>0.05);SP细胞与MM-MSCs共培养后,其Cx43表达均有显著上调(P<0.001);阻断GJ后,SP细胞的Cx43表达则呈现明显下调(P<0.001),详见图2。
-
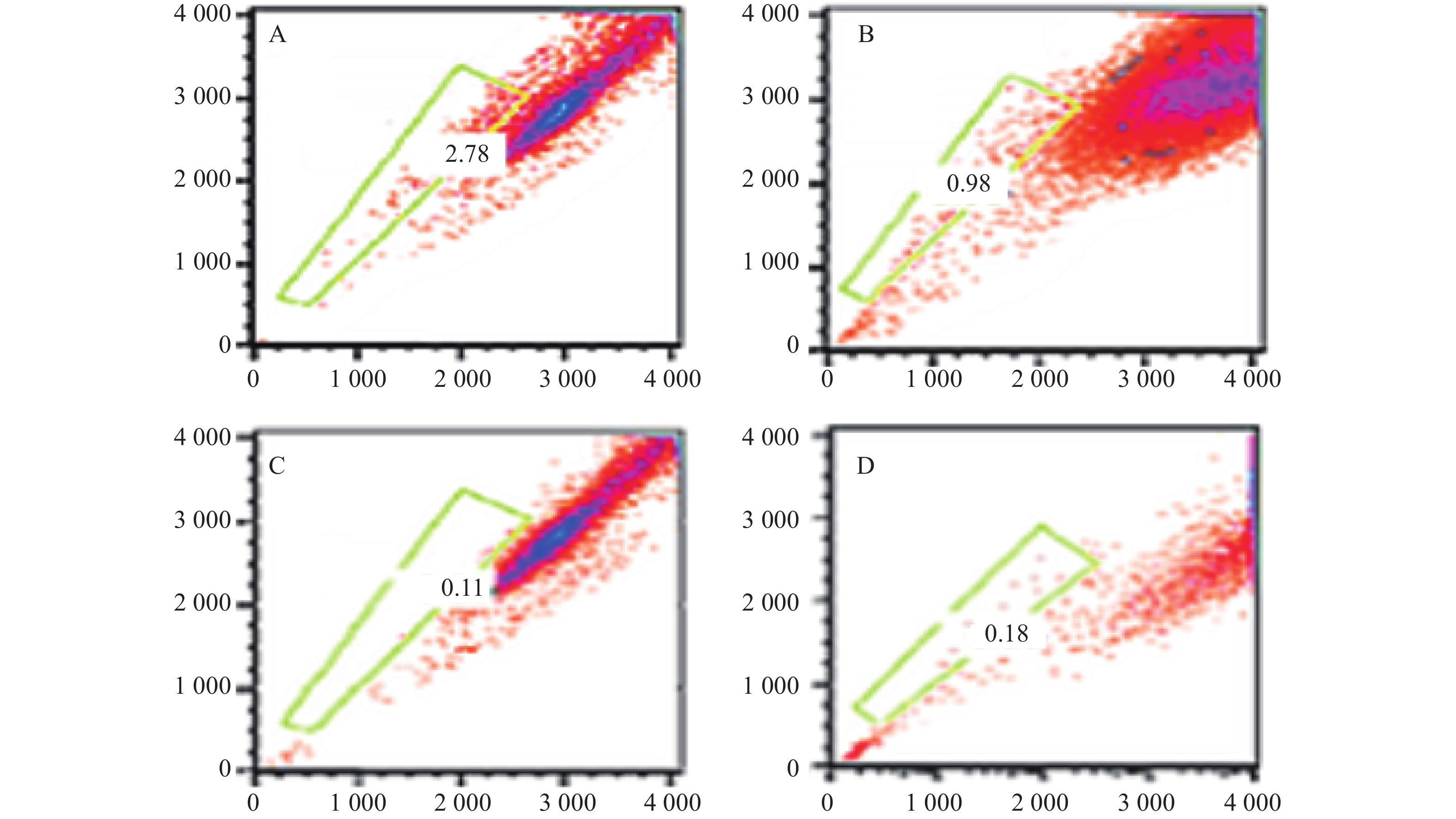
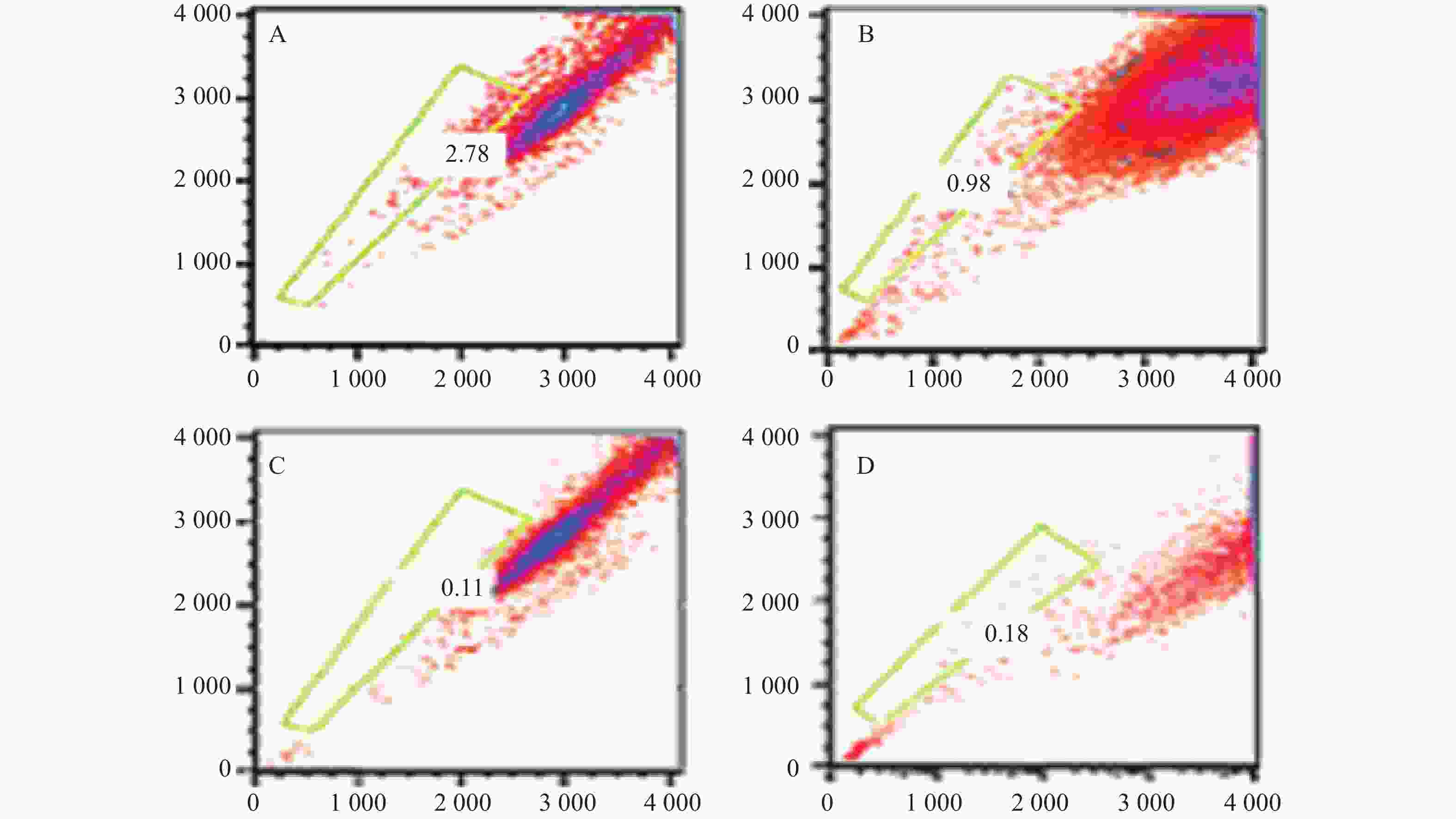
本研究对6例MM患者的原代细胞及4种MM细胞株的检测提示,采用Hoechst 33342染色后应用FCM技术可将MM细胞分为2群,即主群细胞(MP)和侧群细胞(SP)[6]。所有MM细胞均存在不同比例的SP细胞。MM细胞株中SP细胞含量分别为1.783 %、0.8256 %、0.082 %、0.177 %,而原代细胞不具备可重复性,鉴于RPMI 8266细胞中SP细胞含量较多,且稳定,此后实验采用的SP细胞均来自RPMI8226,详见图3。
-
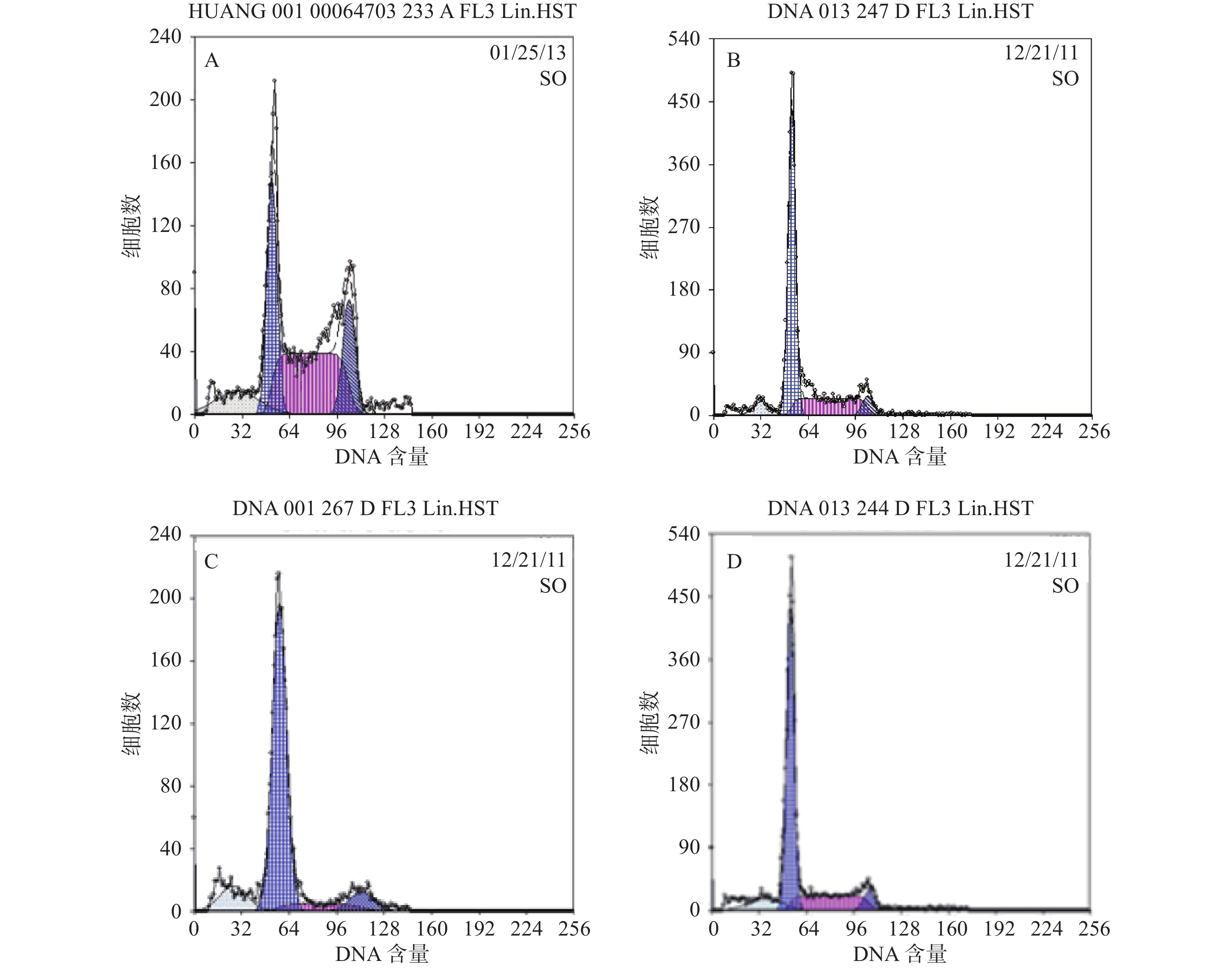
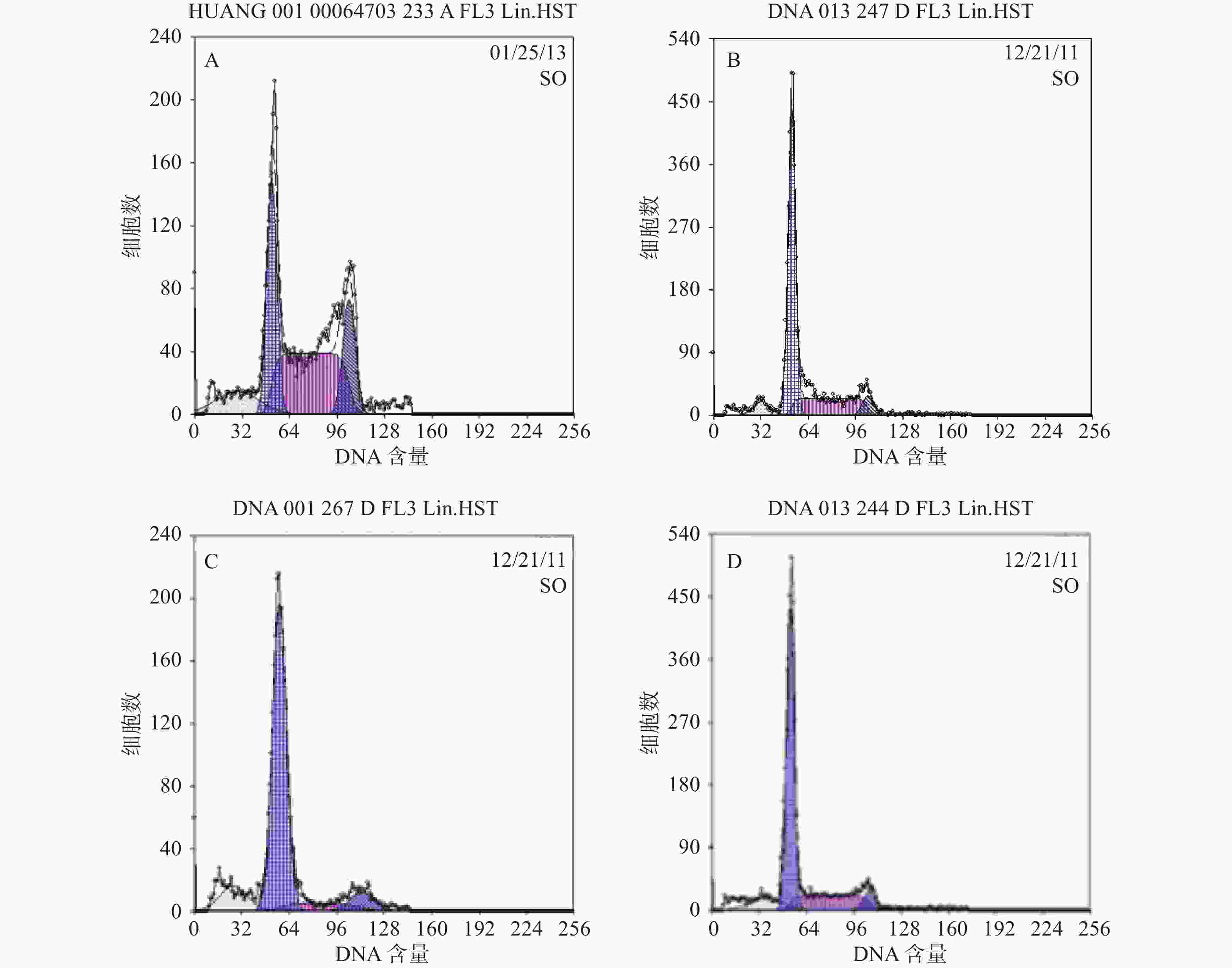
结果分析提示SP亚群中处G0期细胞比例显著高于MP亚群,分别为(44.34±1.7) %和(28.49±1.1) % ,提示SP亚群中包含更多处静止期的MM细胞。与MM-MSCs共培养后发现MM-MSCs具有促进SP亚群细胞进入G0期的作用,其G0期细胞达(82.6±0.1) % (P<0.001),而加入间隙连接抑制剂α-GA后,MM-MSCs对SP亚群的这一作用减弱,细胞进入增殖周期者增多,G0期细胞降至(63.42±3.86) % (P<0.01),详见图4。
-
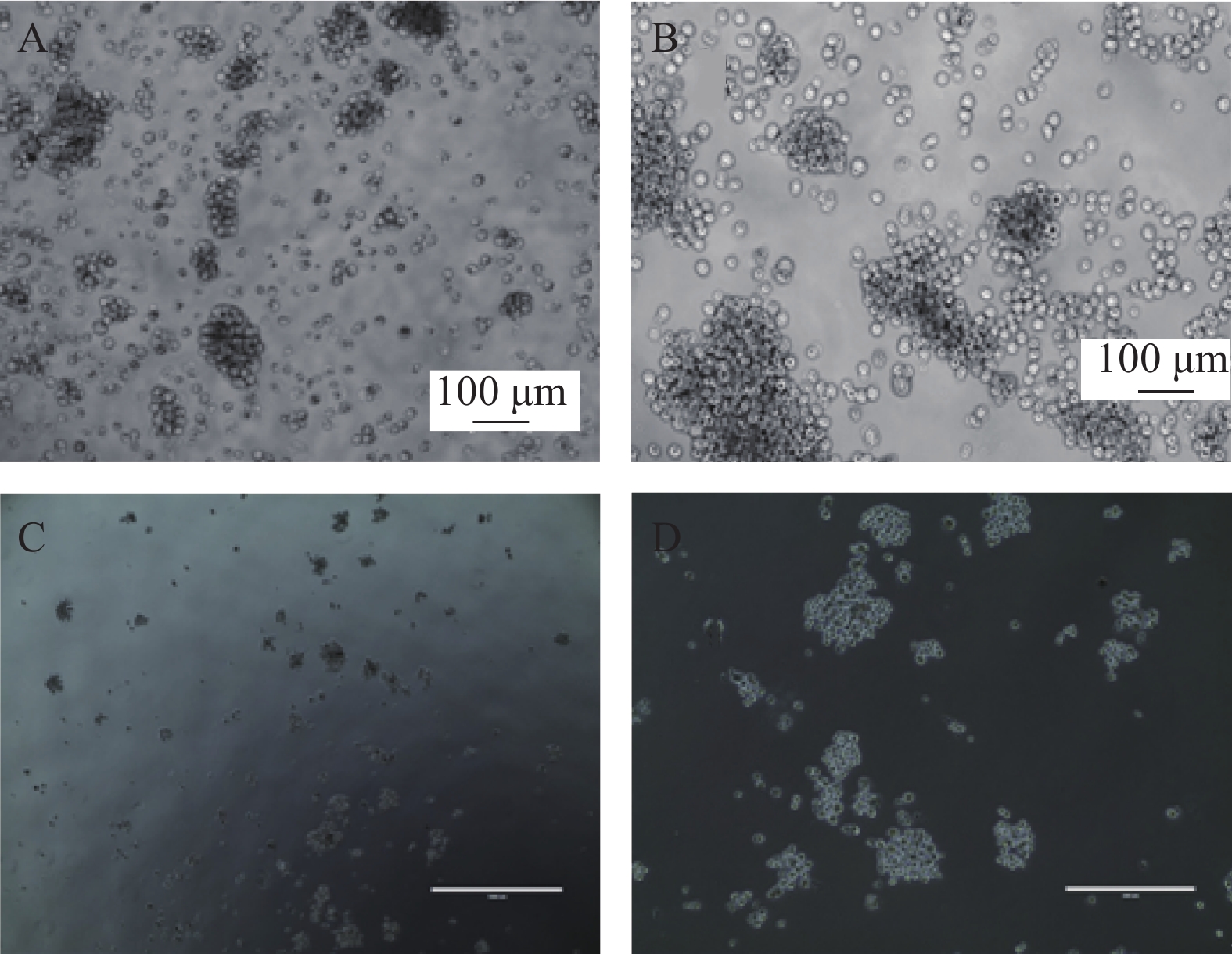
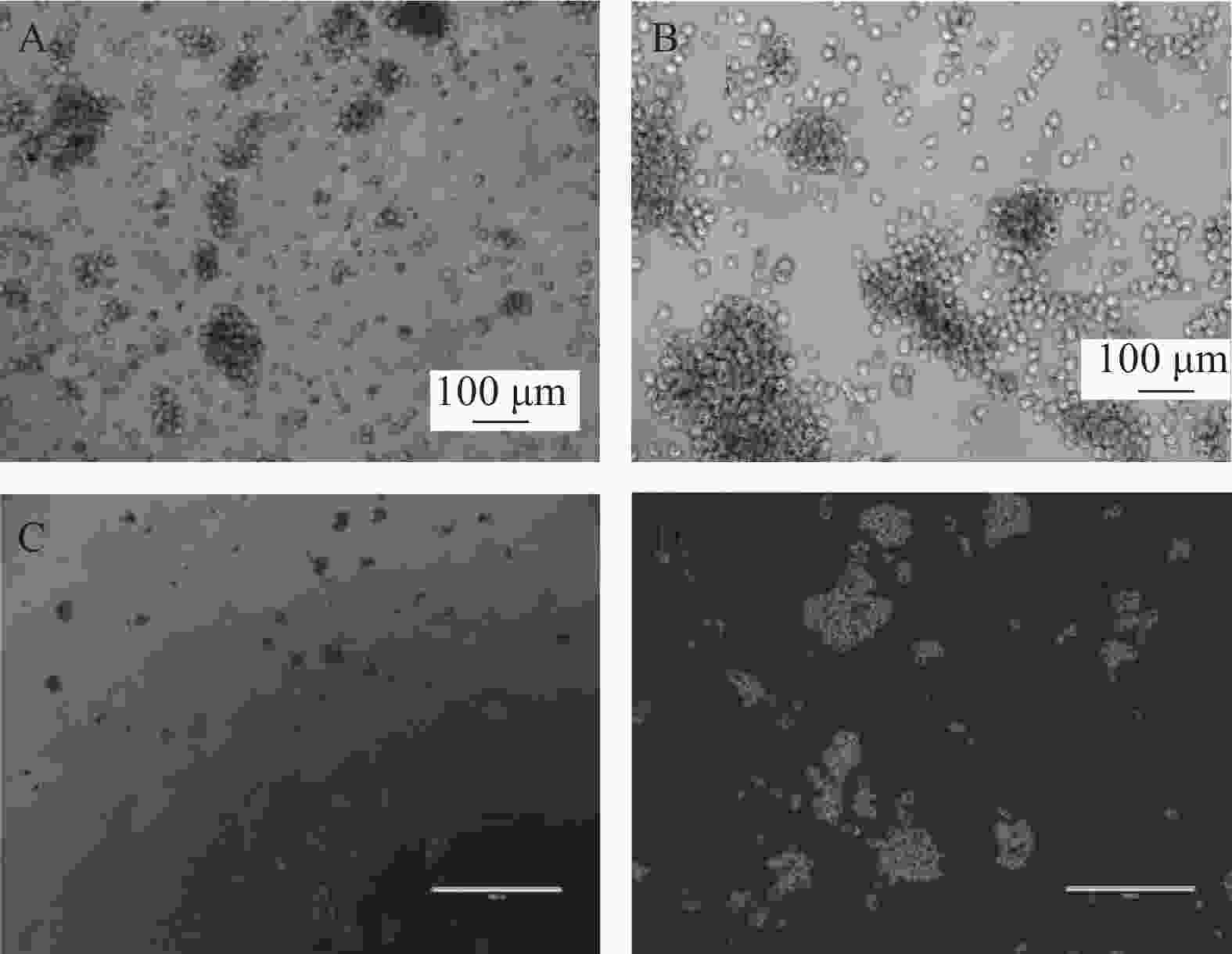
我们利用克隆形成实验分析SP细胞体外形成集落的能力,SP细胞单独培养、与ND-MSCs共培养、与MM-MSCs共培养、与ND-MSCs共培养体系中加入通道阻断剂,与MM-MSCs共培养体系中加入通道阻断剂后单克隆直径、克隆形成数、克隆形成率见表3。结果显示出与MM-MSCs共培养的SP细胞有更强的克隆形成能力。加入通道阻断剂后克隆形成能力均表现出一定程度的下降,单细胞克隆直径减小,克隆形成率降低,见图5。
组别 单克隆直径
(cm)克隆形成数 克隆形成率
(/2000)SP 0.28±0.16 1722±127 86%±6% SP+ND-MSCs 0.33±0.14 1858±89 93%±4% SP+MM-MSCs 0.38±0.21 1900±85 95%±4% SP+ND-MSCs+GA 0.25±0.22 1532±112 77%±6% SP+MM-MSCs+GA 0.31±0.17 1755±76 88%±4% -
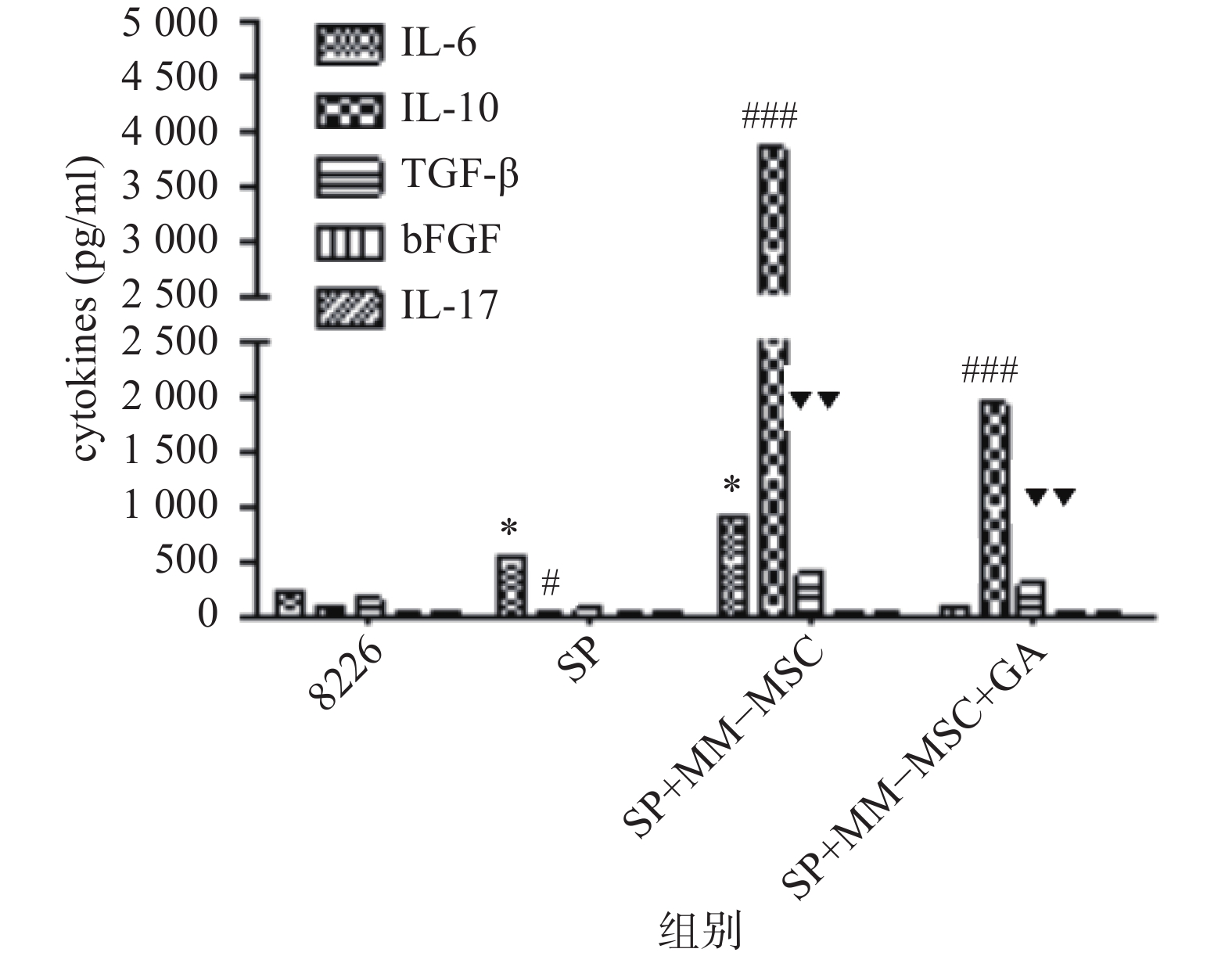
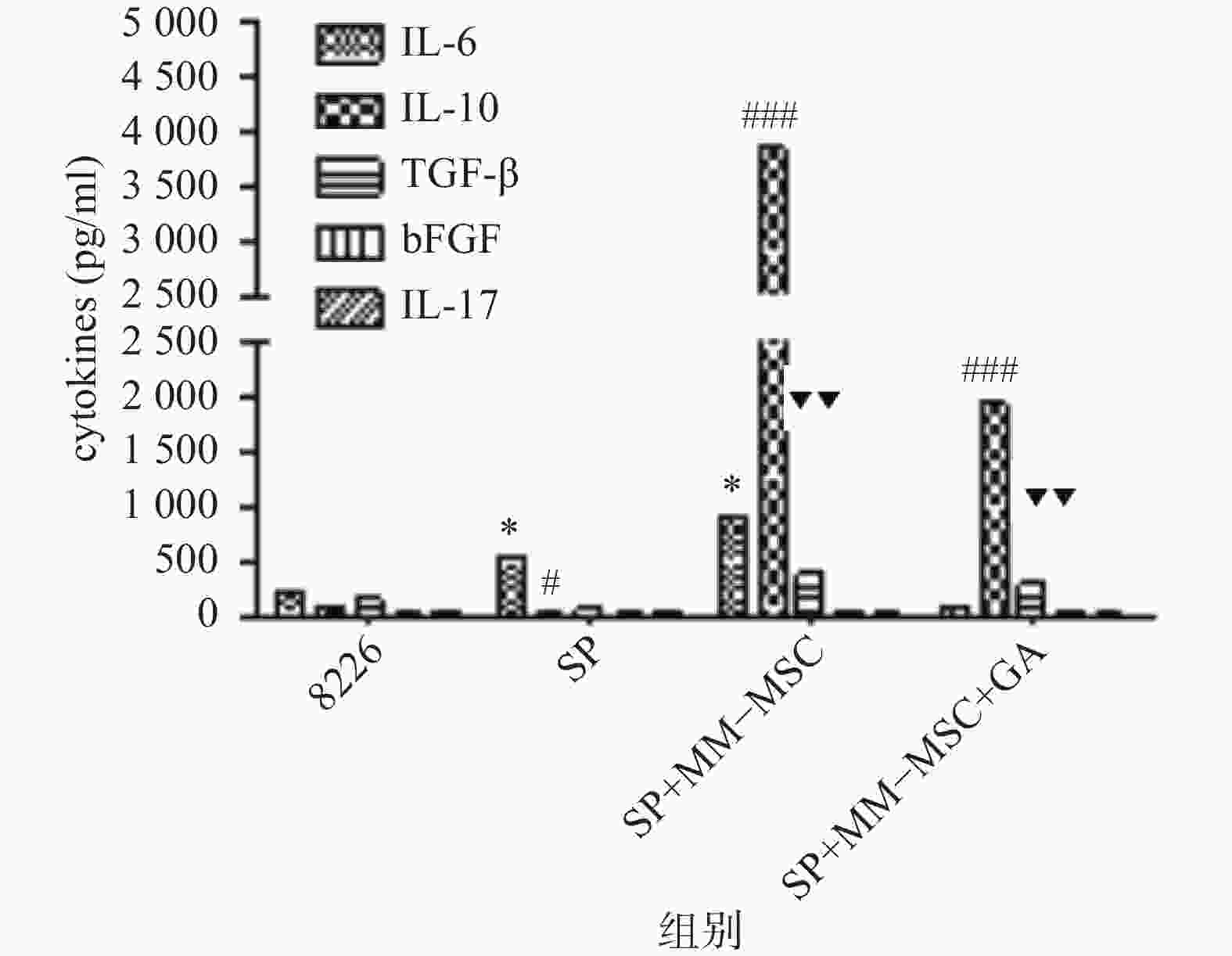
CBA分析显示,MM-MSCs单独培养24 h后,其培养上清中存在高水平的IL-6,较低水平的TGF-β、bFGF和IL-17,基本无IL-10分泌;RPMI 8266细胞培养24 h后上清中可以测得较低水平的TGF-β及少量bFGF、IL-17、IL-6及IL-10;共培养24 h后,其上清中IL-6、IL-10和TGF-β水平较前明显升高(P<0.05),尤其是IL-6和IL-10水平较单独培养时显著升高(P<0.01),bFGF和IL-17共培养前后则无明显变化;加入GJ阻断剂后,细胞因子IL-6、IL-10和TGF-β的分泌有所降低(P<0.05),见图6。
-
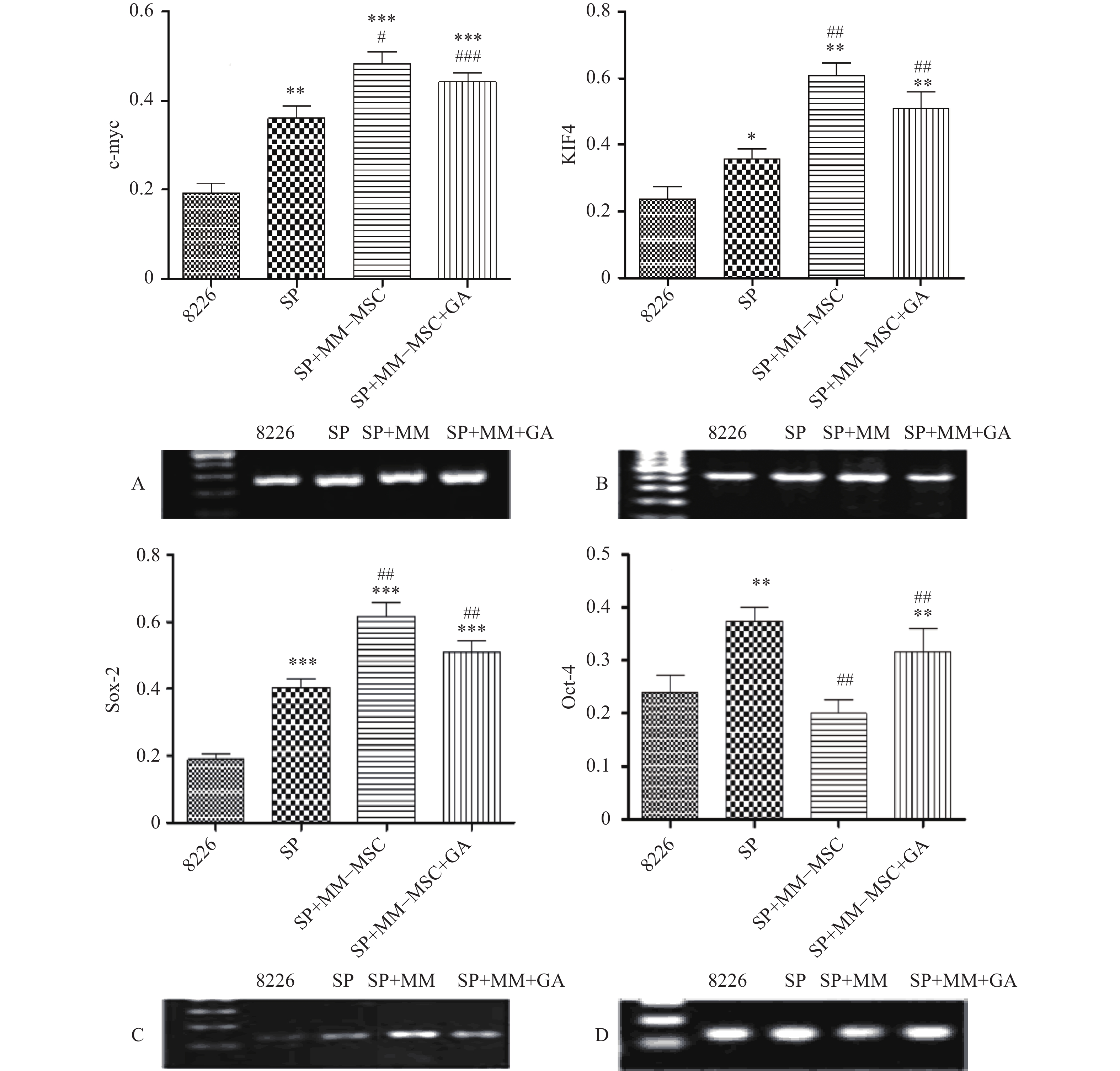
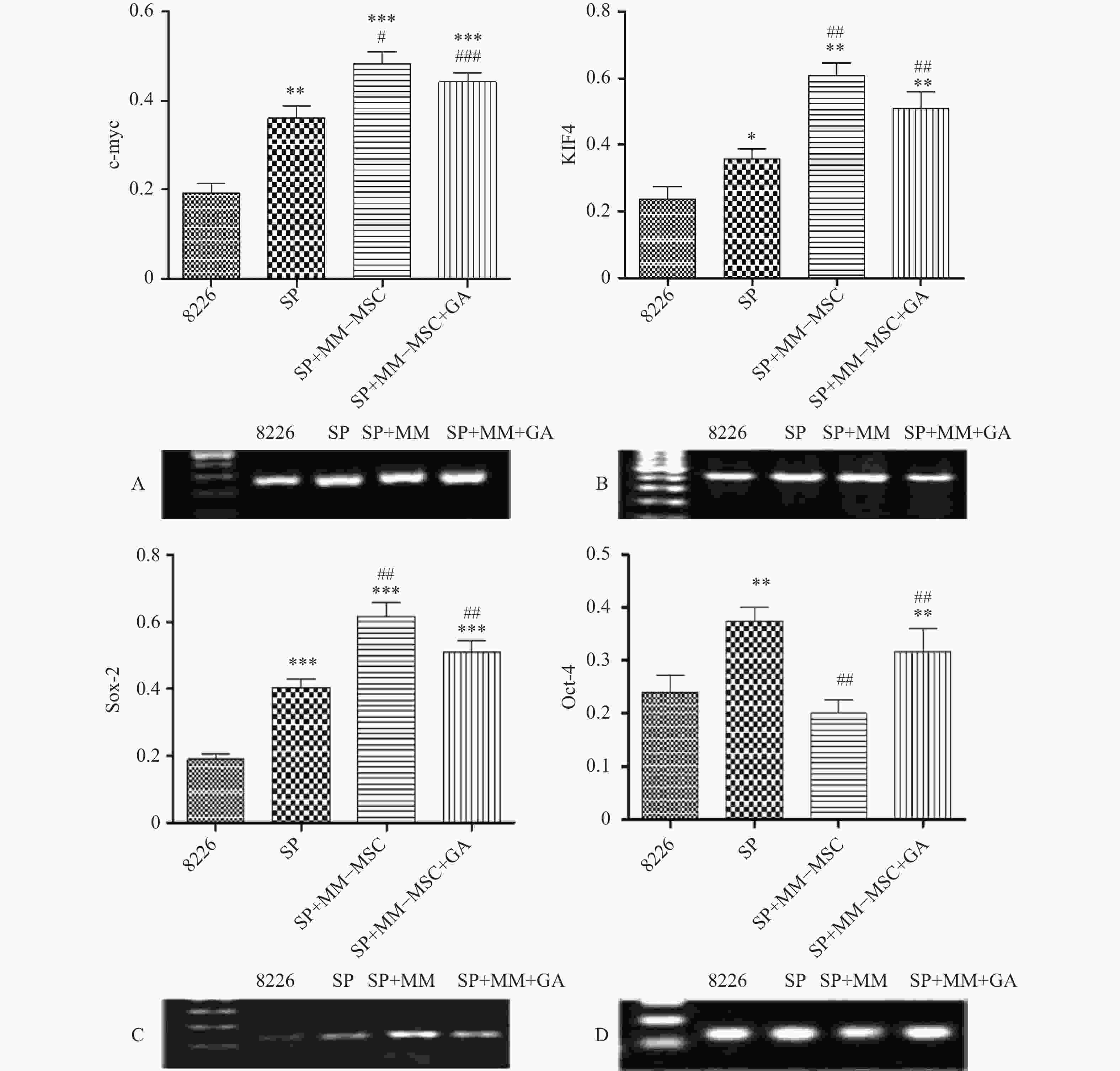
RT-PCR检测发现RPMI8266存在一定量c-myc、KIF4、SOX2和Oct-4基因表达,但SP细胞亚群中该类基因表达明显上调,两者具有显著性差异(P<0.05),将SP细胞与MM-MSC共培养后,可观察到c-myc、KIF4和SOX2基因表达的显著上调(P<0.001),而Oct-4基因表达下调,加入GJ阻断剂后,原上调的基因均有不同程度下调,但无明显区别(P>0.05),见图7。
-
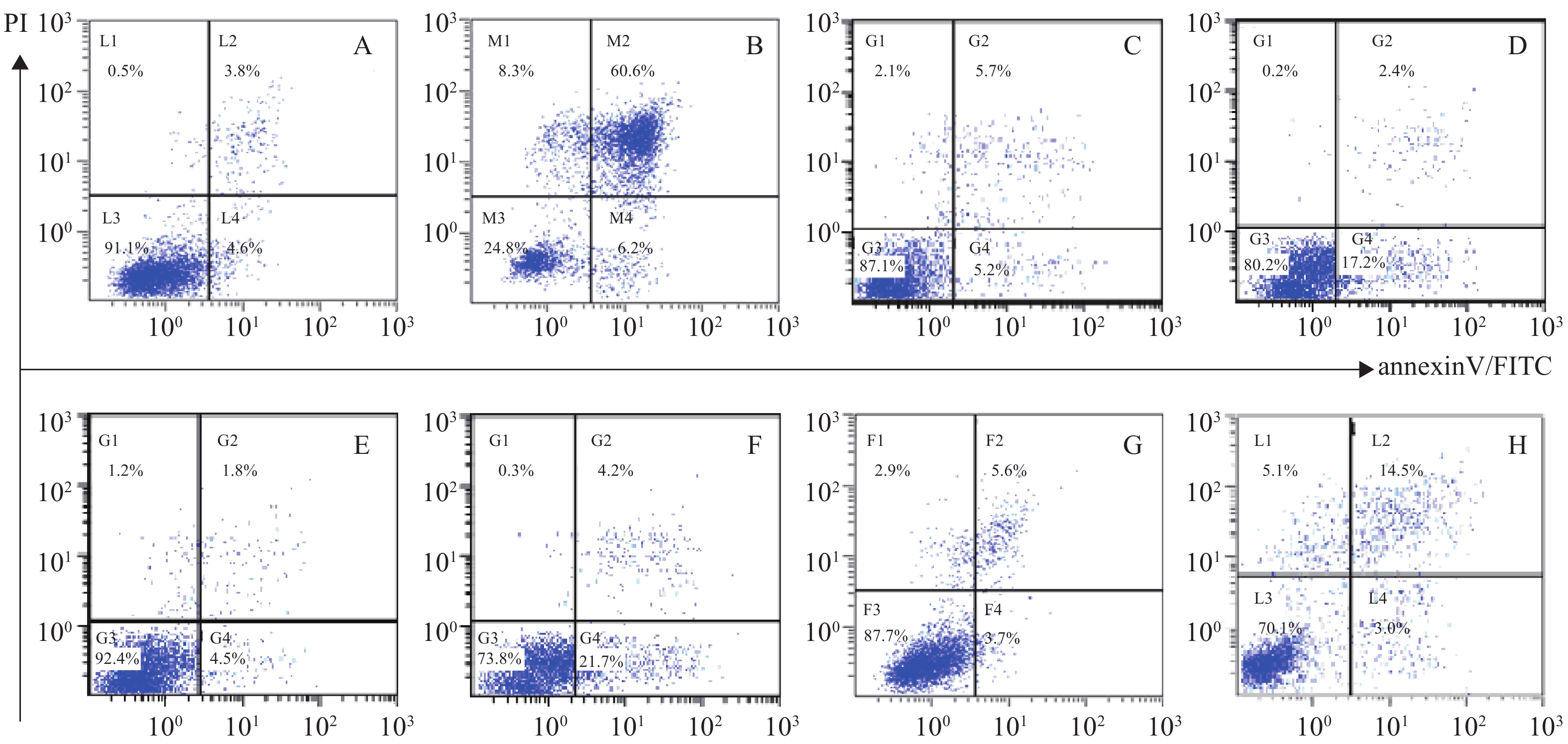
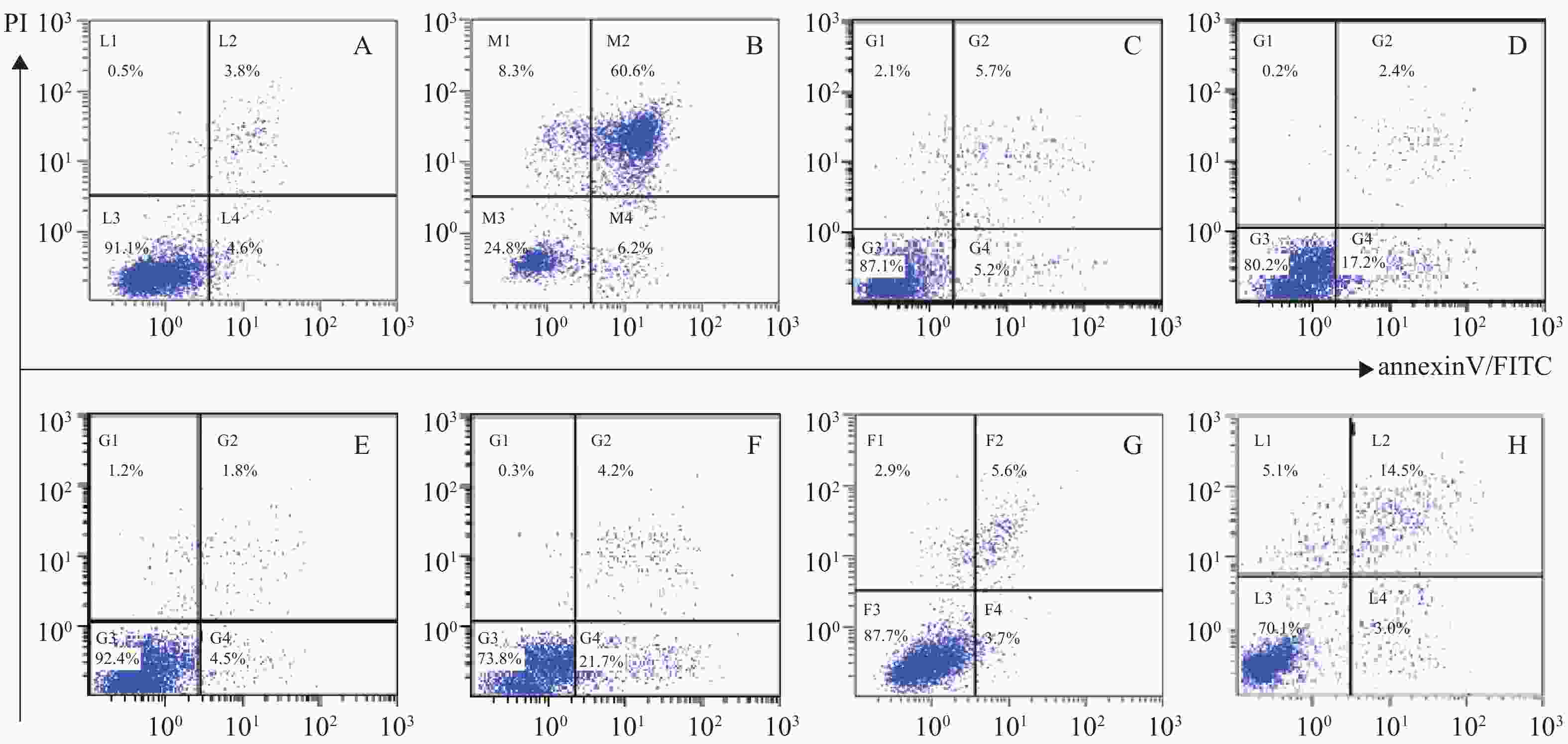
体外PI/Annexin V检测显示,RPMI 8226的MP细胞对BTZ诱导的细胞凋亡敏感,而对SP细胞敏感性较差,其凋亡率分别为(66.8±0.77)%和(25.9±0.86)%,P<0.001。与MM-MSCs直接共培养后,BTZ诱导的凋亡作用较单独培养明显减弱(P<0.05),MM-MSCs具有一定保护作用,加入GJ阻断剂后,可部分恢复MM细胞对硼替佐米的敏感性,证实MM-MSCs可保护骨髓瘤细胞免受抗肿瘤药物影响,而GJIC在其中可能起到一定作用,见图8。
-
MM是一种恶性浆细胞疾病,以肿瘤细胞与骨髓微环境中基质细胞的复杂的相互作用网络为特征,骨髓基质细胞可促进MM细胞的生存、增殖和药物抗性。骨髓间充质干细胞是骨髓基质细胞中最主要的干细胞群体,能够分化成多种细胞系,包括成纤维细胞、脂肪细胞、软骨细胞和成骨细胞。MSCs可以迁移到原发肿瘤和转移部位,这意味着这些细胞可能调节肿瘤的生长和转移。MSCs在与MM细胞相互黏附及作用过程中部分细胞特性发生改变,成为肿瘤相关BMSCs,即MM-MSCs,MM患者来源的骨髓间充质干细胞显示出功能异常,说明骨髓间充质干细胞在骨髓瘤的发生、发展中不是旁观者,它还可分泌多种细胞因子影响MM细胞的生长、生存、耐药、迁移,在疾病的发生、发展中起了重要的作用,目前成为治疗多发性骨髓瘤的新的研究热点[7-8]。
尽管近几年MM的治疗取得了长足的进步,但目前它仍然是不可治愈的,有证据表明MM细胞之间存在异质性,尤其是可能存在MM干细胞亚群,它具有自我更新及原发耐药特性,可能是MM增殖、维持MM表型及导致疾病复发的原因,但其耐药性的机制还没有得到充分的了解[5, 9]。理想的多发性骨髓瘤干细胞(MMSCs)的鉴定应依赖于MMSCs的表型,但至今MMSCs的表型尚未得到正确的定义。Goodell等通过FCM在MM细胞中分离出具有干细胞特性的SP细胞,为拓展肿瘤干细胞的研究提供了思路。目前,SP细胞和ALDH1+已经被用来鉴定MMSCs。MMSCs与骨髓微环境的复杂的相互作用维持着MMSCs的自我更新和生存。然而, MMSCs与周围骨髓微环境相互作用的分子需要进一步确认。
在过去的几年里,连接蛋白的异常表达,特别是Cx43的异常表达已经被证实与癌症复发、转移性扩散和不良预后相关。根据癌症的不同分期和类型,Cx43既可以作为肿瘤抑制因子,也可以作为癌基因、生物标记物,我们需要更好地了解Cx43在肿瘤微环境中如何参与、影响肿瘤形成和进展,从而开发基于Cx43的临床可行疗法。Cx43分子在细胞表面形成通道,可使小分子和一定量大分子物质在细胞间转移,这一特性使它们存在将化疗药物直接送入肿瘤细胞内的潜力,从而成为肿瘤治疗新的非常有吸引力的靶点。
为明确MM-MSCs与SP细胞的相互作用,首先我们采用Hoechst33342标记FCM术检测骨髓瘤细胞株及新鲜MM标本,结果证实所有检测样本中均存在SP细胞亚群,4 种 MM 细胞株检测SP 细胞含量分别为1.783 %、0.8256 %、0.082 %、0.177 %,并成功分选SP细胞比例最高的PRMI8226细胞株的SP细胞。在此基础上,通过对SP亚群细胞的多种干细胞相关基因如c-myc、KIF4、SOX2和Oct-4表达分析,发现其与RPMI 8226细胞有明显不同。直接与MM-MSCs共培养后SP细胞亚群c-myc、KIF4和SOX2基因表达显著上调 (P<0.001),而Oct-4基因表达下调,加入GJIC阻断剂后,c-myc、KIF4和SOX2基因表达尽管有所下调,但并无显著性差异。对于细胞周期的分析也证实SP细胞亚群中处G0期细胞比例显著高于MP亚群,分别为(44.34±1.7)%和(28.49±1.1)% ,提示SP细胞亚群中包含较多处静止期MM细胞,推测与该群细胞化疗敏感性差可能有关。共培养后发现MM-MSCs有促进SP亚群细胞进入G0期的作用(P<0.001),其G0期细胞达(82.6±0.1)%,而加入间隙连接抑制剂α-GA后,这一作用减弱,细胞进入周期者增多,G0期细胞为(63.42±3.86)%。体外集落形成试验证实从MM细胞分离的SP细胞本身具有较强的集落形成能力,但在MM-MSCs存在的前提下其细胞形成的体外集落细胞数更多,胞体较大且细胞折光性强,提示细胞活力较好,培养体系中加入GJ阻断剂后体外集落形成能力均现出一定程度的下降,克隆形成率降低,单细胞克隆直径减小。
前期我们的研究证实MM-MSCs与MM细胞可形成功能性GJIC,并在多发性骨髓瘤的发病中扮演重要角色[2-3, 10],为进一步观察间充质干细胞中连接蛋白Cx43组成的细胞间隙连接在多发性骨髓瘤SP细胞生存及耐药中的作用,我们通过蛋白印迹试验证实除SP细胞基本无Cx43表达外,ND-MSCs和MM-MSCs、MM细胞株及初诊患者MM细胞均表达Cx43分子,与SP细胞相比差别显著(P<0.001)。SP细胞与MM-MSCs共培养后,其Cx43分子表达显著上调(P<0.001),加入α-GA可部分下调SP细胞的Cx43表达(P<0.001)。采用CBA技术检测共培养前后培养液中细胞因子变化,发现在直接共培养时MM-MSCs细胞因子分泌谱发生变化,在原有高水平IL-6的基础上,IL-10和TGFβ水平较前明显增加(P<0.05),尤其是IL-6和IL-10较单独培养时显著增加(P<0.01),bFGF和IL-17在共培养前后水平无明显变化,采用α-GA阻断MM-MSCs与SP细胞间的GJIC后,细胞因子IL-6、IL-10和TGF-β的分泌能力下调(P<0.05)。综上,我们认为MM-MSCs通过多种途径影响SP细胞的生物学特性,增加其干细胞相关基因表达、增加静止期细胞比例、增强其体外集落形成能力、改变细胞因子分泌,而在这一过程中Cx43分子的表达及其形成的GJIC具有重要作用。
蛋白酶体抑制剂BTZ是MM治疗的一线药物,尽管BTZ较目前其他化疗药物更为有效,但原发性或获得性耐药仍是限制其疗效的主要原因,目前对于蛋白酶体抑制剂耐药的机制仍未阐明。本研究发现尽管MP和SP细胞均对BTZ敏感,但SP细胞敏感性较差,可能与其本身极低表达Cx43部分相关,其凋亡细胞分别为(66.8±0.77)%和(25.9±0.86)%,P<0.001,经与MM-MSCs直接共培养后,BTZ诱导的凋亡作用明显减弱(P<0.05),MM-MSCs具有保护作用,而在共培养体系中加入α-GA,可部分恢复MM细胞对硼替佐咪的敏感性,由此证实MM-MSCs促进MM SP细胞的生存,保护MM SP细胞免受抗肿瘤药物影响,GJIC在其中起到一定作用。我们前期实验提示,过表达MM细胞的Cx43可提高MM细胞化疗敏感性,但与MSCs共培养后敏感性降低,肿瘤细胞本身Cx43半通道有可能通过增加癌细胞对化疗药物的通透性来降低耐药性,但与微环境中MSCs相互作用后这一作用产生变化,但具体机制仍在进一步研究中。
本研究发现Cx43及其组成的GJIC不但影响MM细胞的迁移,而且也影响恶性浆细胞的药物敏感性,证实骨髓微环境中Cx43及其组成的GJIC在MM发生、发展中具有重要作用,在细胞因子分泌、肿瘤生长、细胞周期变化、基因表达等多个环节中影响MM细胞的生物学行为,基于我们及其他相关研究结果,我们证实MM细胞与MM-MSCs、造血细胞及细胞外基质通过粘附GJIC介导耐药,阻断肿瘤细胞与微环境的GJIC将有助于提高抗肿瘤效益。
Role of intercellular junctions in the biological behavior of SP cells of multiple myeloma
doi: 10.12206/j.issn.1006-0111.202105104
- Received Date: 2021-05-22
- Rev Recd Date: 2021-12-10
- Available Online: 2022-07-27
- Publish Date: 2022-07-25
-
Key words:
- multiple myeloma /
- gap junction connexin /
- tumor microenvironment
Abstract:
| Citation: | WANG Ziyan, ZHANG Xiaohui, RUI Mingzhong, ZHOU Min, FU Jinxiang. Role of intercellular junctions in the biological behavior of SP cells of multiple myeloma[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(4): 326-334. doi: 10.12206/j.issn.1006-0111.202105104 |







 DownLoad:
DownLoad: