-
骨质疏松症(OP)是一种由骨吸收和骨形成之间的关系失衡造成,以低骨量和骨组织微结构破坏为特征,导致骨质脆性增加和易于骨折的全身性骨代谢疾病。其中,成骨细胞是骨形成的功能细胞,在维持骨稳态中起到关键作用[1]。目前,高氧化应激相关的骨丢失已成为骨质疏松研究领域的热点。有研究表明,细胞保护酶是机体对抗氧化应激状态下活性氧(ROS)损伤的主要机制,其活性主要由转录因子Nrf2和FoxO调控,而二者所介导的氧化应激通路同样被证实具有调节成骨细胞氧化还原平衡以及促进骨形成分化的功能[2]。与此同时,β-淀粉样蛋白(amyloid β-protein,Aβ)的沉积可使机体ROS生成增多,进而抑制成骨细胞的增殖、成骨基质的产生及矿化[3]。由此可见,Aβ沉积偶联的氧化损伤是破坏成骨细胞骨形成,进而引发骨丢失的一大诱因。
啤酒花(Hops, Humulus lupulus L.)为桑科葎草属多年生草质蔓生藤本植物,其雌性球穗花序不仅作为啤酒酿造的添加原料,也是全球广泛应用的植物药,在欧洲广泛用于缓解更年期潮热及绝经后骨质疏松症[4]。我们前期研究发现啤酒花能够促进成骨细胞骨矿化结节的形成,降低活性氧水平,并显著改善APP/PS1转基因小鼠的骨丢失[5-6],但其对外源性Aβ损伤成骨细胞的氧化应激水平及骨形成的影响尚不明确。此外,我们首次确认了氧化应激和Aβ沉积之间的双向关联,及其在老年性骨质疏松症发病中的重要作用[7-8]。故本文拟以Aβ损伤的成骨细胞为模型,以Nrf2和FoxO1两条经典氧化应激相关通路为核心,对啤酒花的抗氧化能力及对骨形成干预作用进行探究。
-
啤酒花药材购于昌吉市山水啤酒花有限公司(产地:新疆昌吉;批号:PJH-01),经海军军医大学药学系生药学教研室辛海量副教授鉴定为啤酒花 Humulus lupulus L.的雌性球穗花序。称取啤酒花药材粉末70 g,加入料液比为1∶15的75%乙醇,回流提取3次,减压浓缩干燥成浸膏,HPLC测定得浸膏中主要成分黄腐酚含量为0.55%[9],使用前配制成相应浓度(生药量/ml)的提取液。
其他试剂及厂家:Aβ1-42寡聚体(上海吉尔);N-乙酰-L-半胱氨酸(NAC,上海碧云天);胎牛血清(Gibco,美国);α-MEM培养基等细胞培养试剂(天津灏洋);碱性磷酸酶(ALP)染色试剂盒(南京建成);I型胶原酶(COL-I)、骨桥蛋白(OPN)、核因子-E2-相关因子(Nrf2)、血红素加氧酶-1(HO-1)、NAD(P)H:醌氧化还原酶(NQO1)、细胞叉头框蛋白O1(FoxO1)、超氧化物歧化酶(SOD-2)抗体(Abcam,英国)。
-
新生24 h Wistar大鼠,购自上海斯莱克实验动物有限公司[合格证号:2013001831722;许可证号:SYXK(沪)2017-0004]。所有动物实验均符合实验动物伦理学要求。采用二次消化法从新生大鼠颅盖骨分离得到原代成骨细胞,用含10%胎牛血清的α-MEM培养液进行培养,取3~4代成骨细胞进行后续实验分析。
-
取3~4代成骨细胞计算其数目,配制成细胞浓度为1×104个/ml细胞悬液接种于96孔板,根据前期实验结果[9]设置分组:空白对照组,模型组(40 μmol/L Aβ),阳性对照组(NAC, 2.5 mmol/L),啤酒花提取物低剂量组(HLE, 4 μg/ml)、中剂量组(HLE, 20 μg/ml)、高剂量组(HLE, 100 μg/ml),每组设置4个复孔。24 h后按照上述分组更换为含药培养液。给药48 h后采用MTT法检测成骨细胞的增殖情况。
取3~4代成骨细胞计算其数目,配制成细胞浓度为5×104个/ml细胞悬液接种于24孔板。24 h后分别更换为含药培养液(给药浓度同上)。培养过程中每3 d更换1次含药培养液。第8天裂解细胞,收集细胞裂解液,于4 ℃、13 800×g 离心5 min。用对硝基苯磷酸二钠法测定细胞ALP活性[10]。参照ALP染色试剂盒说明书对成骨细胞进行染色。
-
取3~4代成骨细胞计算其数目,配制成细胞浓度为2×105个/ml细胞悬液接种于6孔板。24 h后分别更换为含药培养液(给药浓度同上)。给药48 h后收集各孔中培养基上清液,加入200 μl 0.25%胰蛋白酶消化30 s,离心并重悬,参照凋亡检测试剂盒装载探针,室温避光孵育5 min后,用流式细胞仪进行凋亡率检测。
-
取3~4代成骨细胞接种于6孔板,24 h后分别更换为含药培养液(给药浓度同上)。给药48 h后进行细胞裂解,提取细胞总蛋白,根据BCA试剂盒进行蛋白定量。蛋白变性后进行十二烷基硫酸钠-聚丙烯酰胺凝胶电泳分离,转膜、封闭后,分别加入COL-I、OPN、Nrf2、HO-1、NQO1、FoxO1及SOD-2抗体,4 ℃孵育过夜。用洗膜缓冲液(Tris-buffered saline/Tween-20,TBST)洗膜3×10 min,加入二抗,室温孵育30 min。用TBST再次洗膜3×10 min,采用ECL试剂进行检测。采用Tanon Image软件对蛋白印迹进行半定量分析。
-
取3~4代成骨细胞配制成浓度为2×104个/ml细胞悬液接种于无菌激光共聚焦皿中。24 h后分别更换为含药培养液(Aβ, 40 μmol/L HLE, 100 μg/ml)。给药48 h后用4%的多聚甲醛固定细胞30 min,PBS浸洗后用0.5% Triton X-100(PBS配制)通透20 min,并用5% BSA封闭液室温封闭1 h。先后加入FoxO1抗体及荧光二抗(TRITC标记山羊抗兔抗体)进行孵育,加入DAPI染液避光孵育10 min进行染核。最后洗去多余的DAPI染液,加入少量PBS使细胞保持湿润,并置于荧光显微镜下观察采集图像。
-
实验结果以均值±标准差(
$ \bar x$ ±s)表示。采用SPSS 22.0软件进行数据分析,选用单因素方差分析(One-Way ANOVA)进行组间变量的比较分析。 -
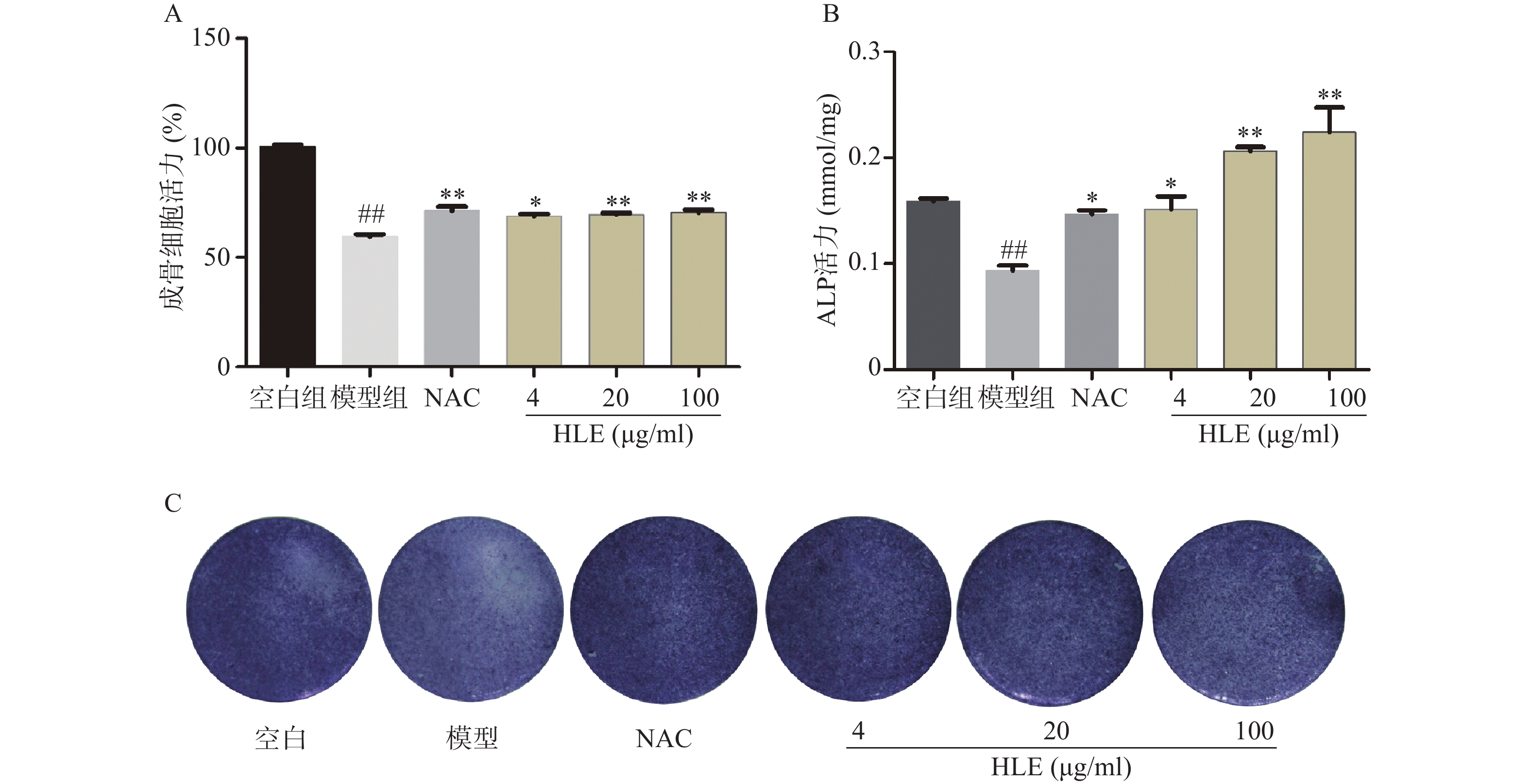
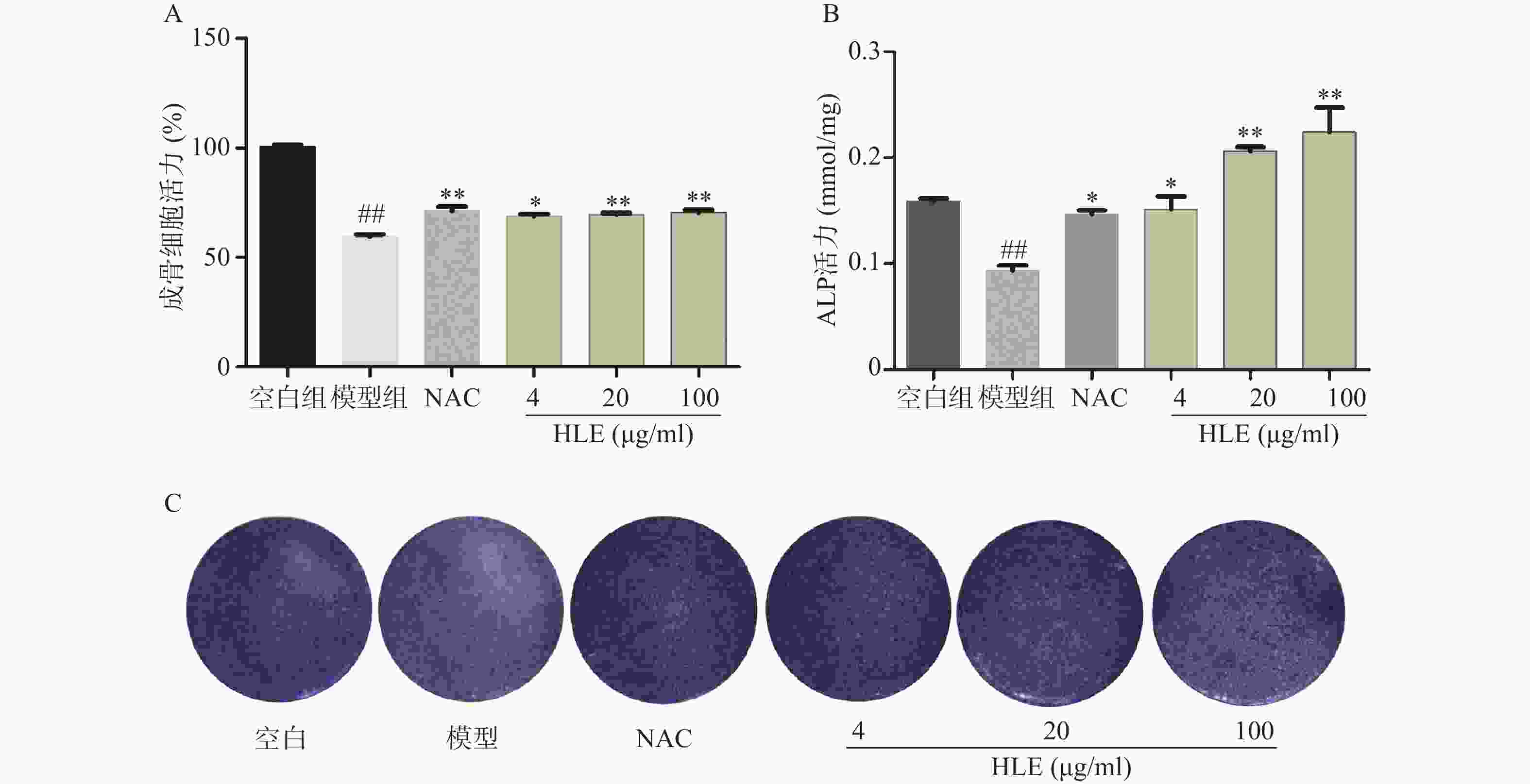
如图1A所示,与空白组相比,Aβ损伤成骨细胞后,其增殖能力显著降低。药物治疗后,低、中、高剂量的啤酒花提取物均可显著促进Aβ损伤成骨细胞的增殖。另一方面,与空白组比,Aβ显著降低了成骨细胞的ALP活性,而啤酒花提取物显著逆转了Aβ损伤成骨细胞的ALP活性,促进成骨细胞的分化(图1B-C)。
-
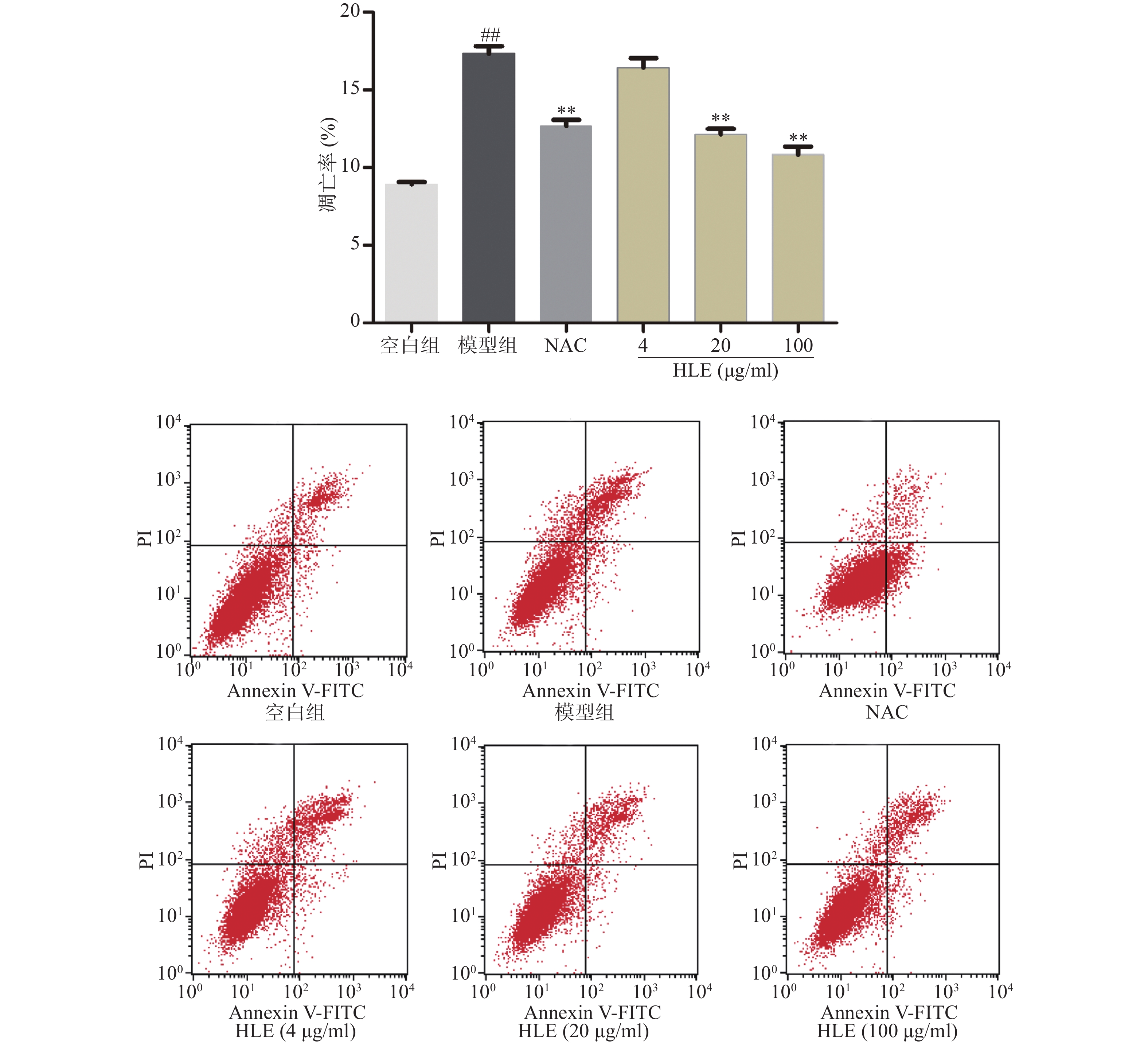
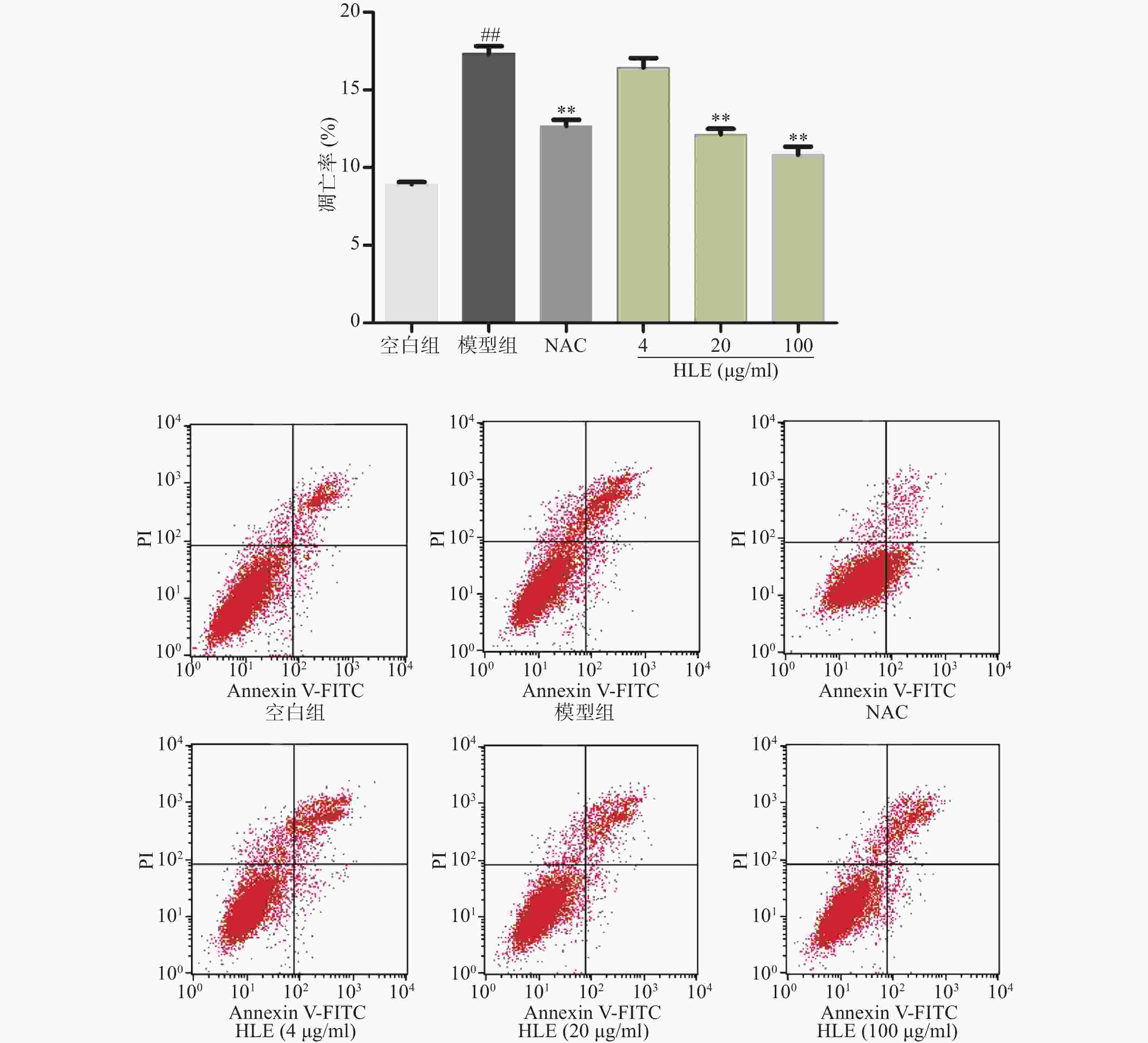
如图2所示,与空白组相比,Aβ损伤提高了成骨细胞的凋亡率,而啤酒花提取物(20,100 μg/ml)可显著抑制Aβ损伤成骨细胞的凋亡。提示啤酒花提取物对Aβ诱导的成骨细胞具有较强的抗凋亡作用。
-
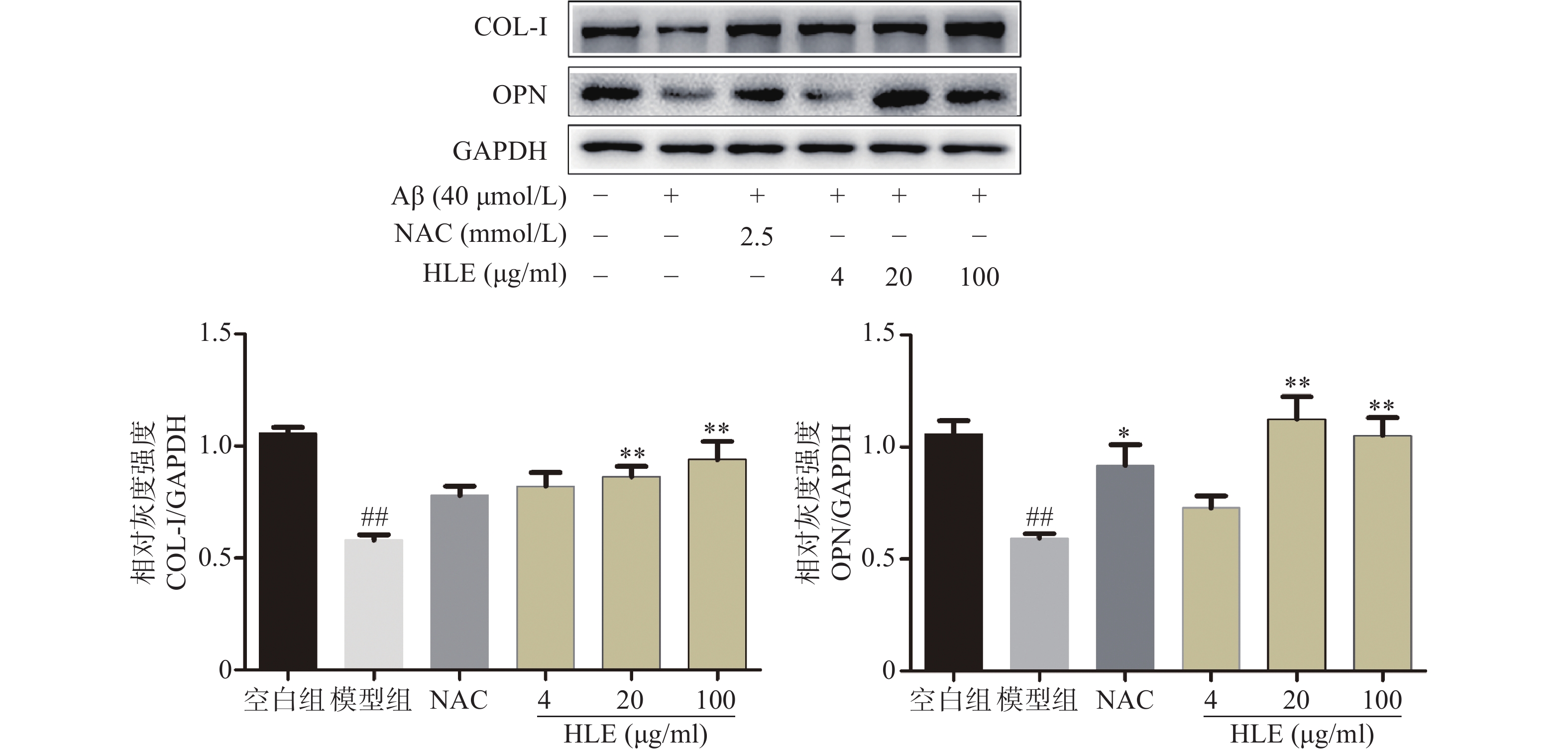
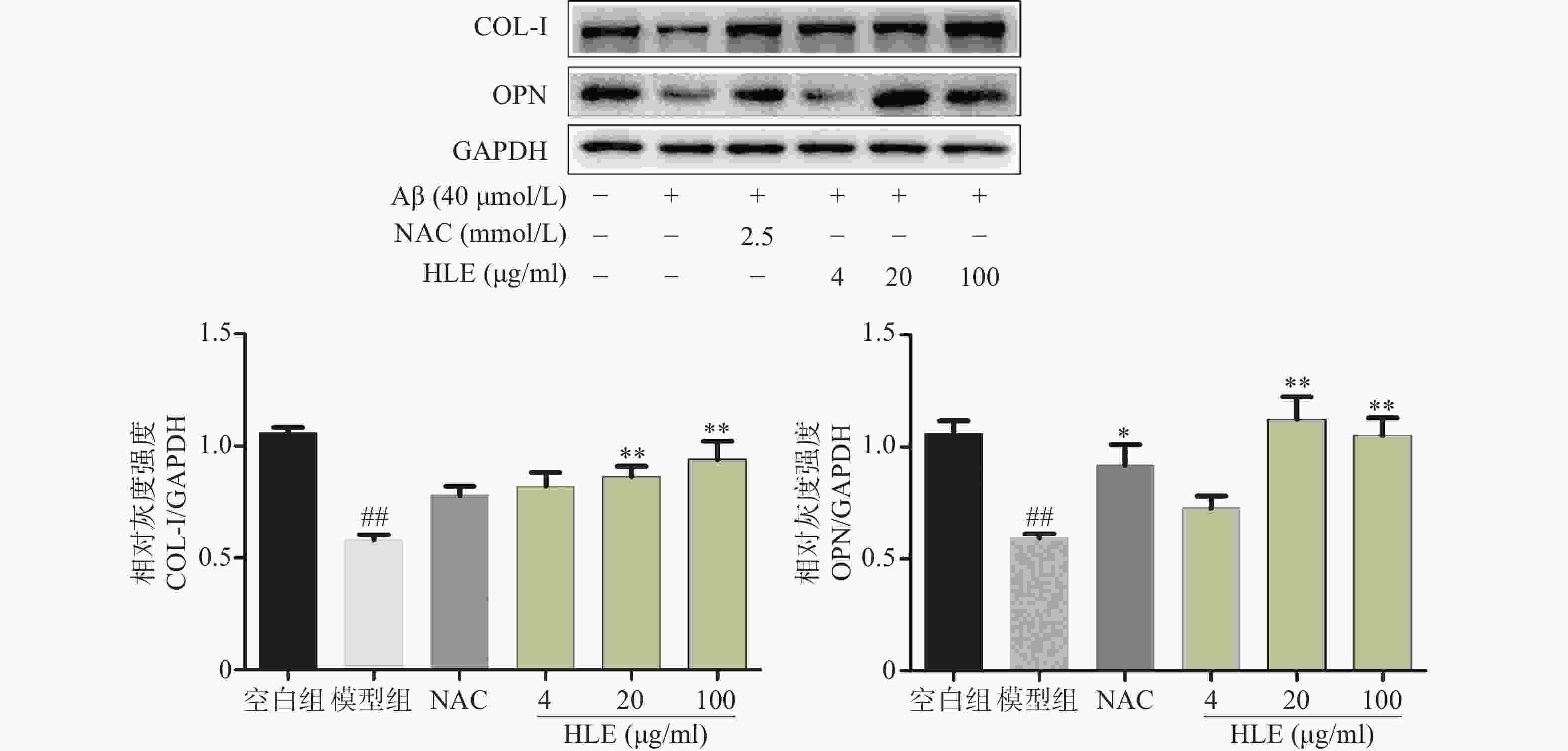
如图3所示,Aβ损伤成骨细胞后,细胞中骨形成相关蛋白COL-I和OPN的表达显著降低。给予啤酒花提取物(20,100 μg/ml)干预后,COL-I及OPN的表达显著增加,提示啤酒花可显著促进Aβ损伤成骨细胞的骨形成。
-
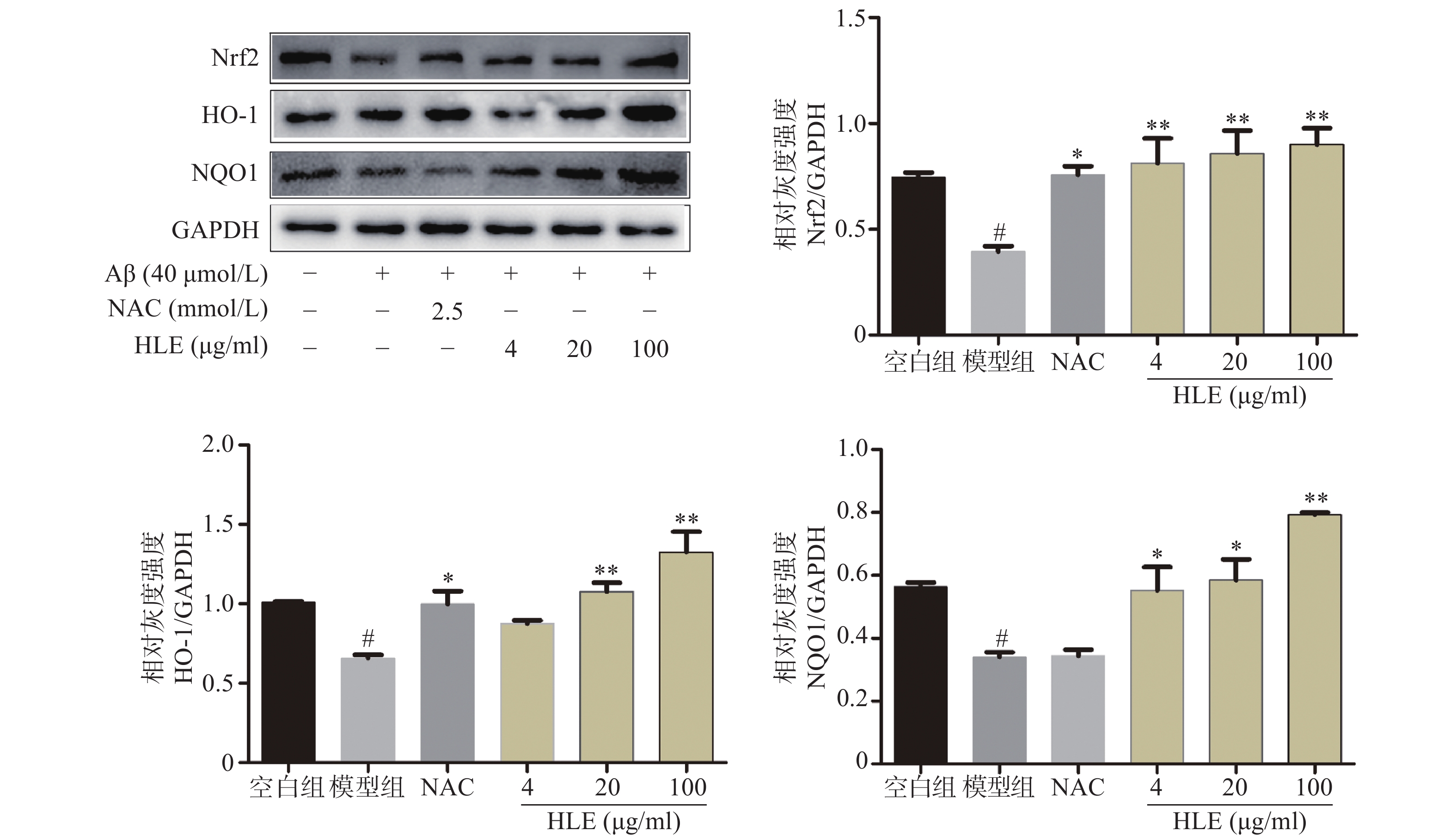
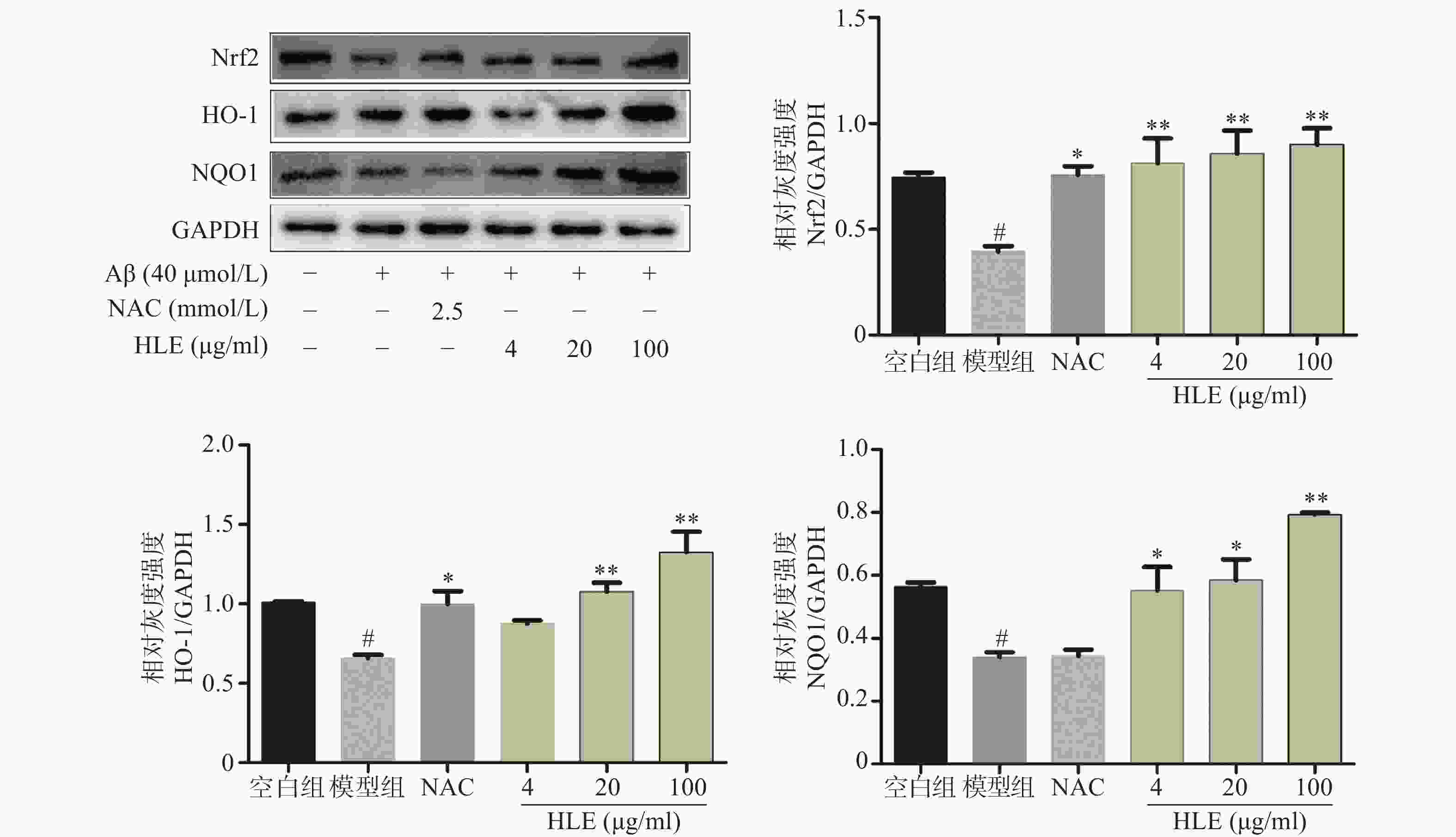
采用Western blot分析Aβ和啤酒花提取物对成骨细胞中Nrf2及其下游抗氧化酶HO-1和NQO1的影响。结果显示,Aβ显著抑制了成骨细胞中Nrf2及其下游蛋白HO-1和NQO1的表达;而低、中、高剂量的啤酒花提取物均可显著促进Aβ损伤成骨细胞中Nrf2、HO-1及NQO1的表达(图4),提示其能够通过介导Nrf2信号通路发挥抗氧化作用。
-
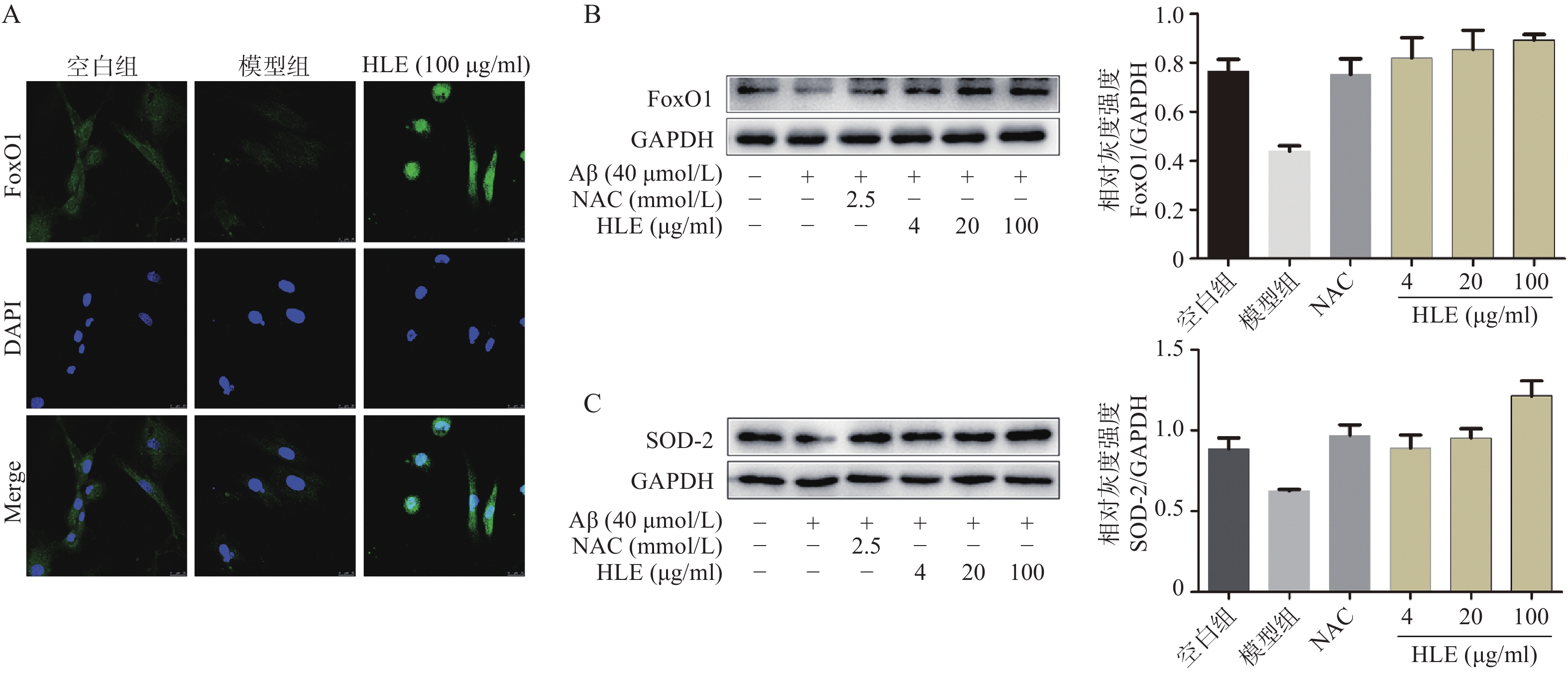
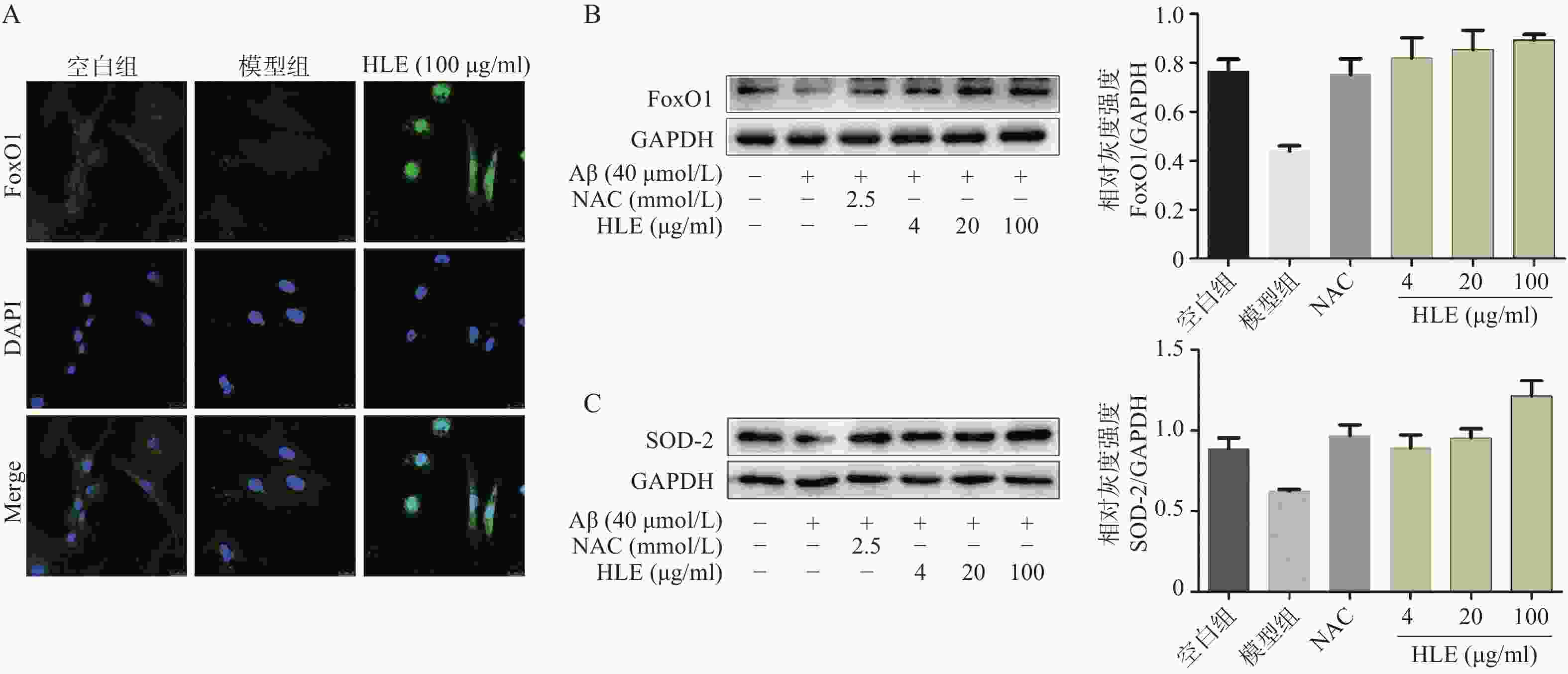
采用免疫荧光及Western blot分析Aβ和啤酒花提取物对成骨细胞中FoxO1信号通路的影响。结果显示,与空白组相比,Aβ显著降低了成骨细胞中的FoxO1含量,而啤酒花提取物(100 μg/ml)可显著下调FoxO1的表达,且使其更多聚集在细胞核内(图5A)。此外,啤酒花提取物(4、20、100 μg/ml)还可显著促进Aβ损伤成骨细胞中FoxO1及其下游蛋白SOD-2的表达,提示啤酒花能够通过介导FoxO1信号通路发挥抗氧化作用。
-
成骨细胞是骨形成的主要功能细胞,在骨形成过程中经历增殖、分化、矿化和凋亡四个阶段。其中,成骨细胞的增殖水平反映骨形成的强弱,其分泌的ALP是成骨细胞分化阶段的关键酶[11],可介导骨组织矿化。在骨形成相关蛋白中,COL-I是骨细胞外基质的主要成分之一,约占骨总蛋白的80%[12],而OPN是一种骨基质糖蛋白,能够促进成骨细胞的黏附和分化[13],二者均为成骨细胞分化成熟的标志。此外,Aβ沉积会引起机体的氧化损伤,在骨代谢中可降低骨髓间充质干细胞向成骨细胞的分化,破坏成骨细胞的活性和功能,抑制骨形成[14]。我们前期研究同样发现过量的Aβ在聚集过程中会产生大量的活性氧,使机体处于高氧化应激状态,反过来又刺激Aβ产生和聚集,形成Aβ与氧化损伤相偶联[15]。该机制在老年性骨质疏松症发病中处于特别重要的位置,并受到学界关注[16]。本研究中,啤酒花提取物可显著逆转Aβ损伤所致的成骨细胞增殖水平下降、ALP活性降低,以及COL-I和OPN的低表达,表明其可显著促进成骨细胞的成熟分化;并抑制Aβ损伤成骨细胞的凋亡,表明其可显著改善Aβ沉积所致成骨细胞的活性损伤,促进骨形成,在维持骨稳态中发挥重要作用。
Nrf-2信号通路是典型的抗氧化通路,能够拮抗各种原因引起的氧化应激。激活Nrf2信号通路不仅能够抑制氧化损伤成骨细胞的凋亡,促进骨形成[17],而且能够拮抗Aβ诱导的细胞损伤[18-19]。应激状态下,Nrf-2转移入核内,与基因中的抗氧化反应元件结合,启动下游Ⅱ相代谢酶基因的表达和转录,以增加细胞对氧化应激的抵抗作用,使细胞免于凋亡[20]。FoxO1为调节成骨细胞氧化还原平衡和成骨功能的主要转录因子,其入核可激活下游SOD-2抗氧化酶,调控细胞内的氧化还原平衡,并促进成骨细胞的增殖与分化,在骨代谢中同样发挥着重要作用[20]。本研究结果表明,啤酒花可激活Aβ损伤成骨细胞的Nrf-2和FoxO1信号通路,促进该氧化应激信号通路中相关蛋白的表达,增加成骨细胞对氧化应激的抵抗作用,使其免于凋亡,进而促进成骨细胞增殖与分化。提示啤酒花具有通过抗氧化而调控骨代谢、维持骨稳态之应用潜力。
Hops extract alleviates Aβ-injury to osteoblasts through antioxidant pathway
doi: 10.12206/j.issn.1006-0111.202103018
- Received Date: 2021-03-17
- Rev Recd Date: 2021-06-01
- Available Online: 2021-12-27
- Publish Date: 2021-11-25
-
Key words:
- Hops (Humulus lupulus L.) /
- Aβ /
- osteoblast /
- antioxidation /
- osteoporosis
Abstract:
| Citation: | XIA Tianshuang, LIU Xiaoyan, JIANG Yiping, LI Xiaojin, WANG Guoping, XIN Hailiang. Hops extract alleviates Aβ-injury to osteoblasts through antioxidant pathway[J]. Journal of Pharmaceutical Practice and Service, 2021, 39(6): 509-514. doi: 10.12206/j.issn.1006-0111.202103018 |








 DownLoad:
DownLoad: