-
针对海训需求,本课题组研制出一款能同时满足防晒和防水母蛰伤需求的防水型多效防护乳,对于增强海军部队日常海训强度和提高海军战斗力具有重要作用和意义。经文献和相关专利调查,以及初步药效学评价[1-5],本课题确定以LaCl3·7H2O (500 mmol/L)、MgCl2·6H2O(25 mmol/L)、CaCl2(25 mmol/L)的水溶液作为防水母蛰伤成分加入到基质处方中。镧是一种重要的稀土元素,广泛应用与电子、医药、生物医学等领域,有文献报道[6-9],镧离子暴露可能会导致人的健康问题,对阈值效应而言,需要进行未观察到有害作用剂量(NOAEL)的测定。防护乳长期、大量涂抹,而且很可能接触破损的皮肤,镧离子(La3+)可能通过经皮吸收进入人体体循环,而其在体内的毒性或潜在毒性均未明确。此外,迄今为止,未见含镧乳膏的透皮安全性的报道,所以必须对防护霜中添加的镧离子进行安全性验证。
电感耦合等离子体发射光谱技术具有分析速度快、线性范围宽、可多元素同时测定、检出限低等特性,已被广泛地应用于各类物质中无机元素的分析检测[10]。本文以镧元素为检测对象,结合微波消解与电感耦合等离子体发射光谱(ICP-OES)技术,建立一种体内含量分析方法,可为多效防护乳的质量分析和透皮安全性评价提供基础数据。
HTML
-
Thermo Scientific iCAP6000系列电感耦合等离子体光谱仪,包括RF发生器、光谱仪系统和光电转换检测器、iTEVA工作站(美国赛默飞世尔公司);MDS-6G多通量微波消解/萃取系统(上海新仪微波化学科技有限公司);AL-104电子天平(瑞士梅特勒公司);VORTEX-6涡旋振荡器(海门市其林贝尔仪器有限公司);Barnstead D3750超级纯水仪(美国赛默飞世尔公司)。
-
硝酸(优级纯,批号:20190920)购自国药集团化学试剂有限公司;La元素标准溶液(浓度1000 μg/ml)购自国家有色金属及电子材料分析测试中心;水为超纯去离子水(≥18.2Ω);多效防护乳(批号:82004163)为中试放大样品。
-
SD大鼠,均为雄性,体重约250 g,昭衍(苏州)新药研究中心有限公司提供,许可证号(苏)2018-0006。
1.1. 仪器
1.2. 药品与试剂
1.3. 实验动物
-
给药前,尽可能剃掉大鼠背部及两侧体毛,约5 cm×10 cm,休息观察24 h。实验前禁食12 h,自由饮水。实验前,将大鼠编号,用丙酮轻轻擦去皮脂。然后以0.4 g/cm2的剂量分别将防护乳均匀涂布在大鼠裸露皮肤表面。分别于给药前和给药后1 h在眼眶静脉丛取血1ml,置于肝素化的离心管中,于−20 ℃条件下保存。
-
iCAP6000系列电感耦合等离子体光谱仪最佳化后工作条件为:射频功率1150 W;采样深度5 mm;冷却气流量12 L/min;辅助气流量0.5 L/min;泵转速45 r/min。样品冲洗时间30 s;样品测定次数3次。微波消解仪参数和检测程序如表1所示。
步骤 温度(T/℃) 保持时间(t/min) 功率(P/W) 1 120 6 800 2 150 5 800 3 180 15 800 -
准确吸取血样1 ml,置于聚四氟乙烯烧杯中,加入硝酸8 ml,混匀,在电热板上于120 ℃加热预消解20 min,血样可完全溶解为黄色的消解液;然后,按照表1程序将样品置于微波消解仪中完全消解。取出样品置于160 ℃的电热板上加热赶去硝酸,消解液呈无色透明或略带黄色,直到四氟乙烯杯剩余溶液小于1 ml时,将消解液转移至10 ml量瓶中,用2%硝酸溶液洗涤容器,洗液合并于量瓶中,用2%硝酸溶液稀释至刻度,摇匀,得到供试品溶液。未经给药处理的血样经过上述消解程序得到的溶液即为空白基质溶液。
-
精密量取La元素标准溶液1 ml,置10 ml量瓶中,用2%硝酸溶液稀释至刻度,得到浓度为100 μg/ml的储备液(A)。精密量取储备液0.05、0.1、0.25、0.5、1、2.5 ml,分别置50 ml量瓶中,用2%硝酸溶液稀释至刻度,摇匀,得到浓度分别为0.1、0.2、0.5、1、2、5 μg/ml的一系列对照品溶液。
-
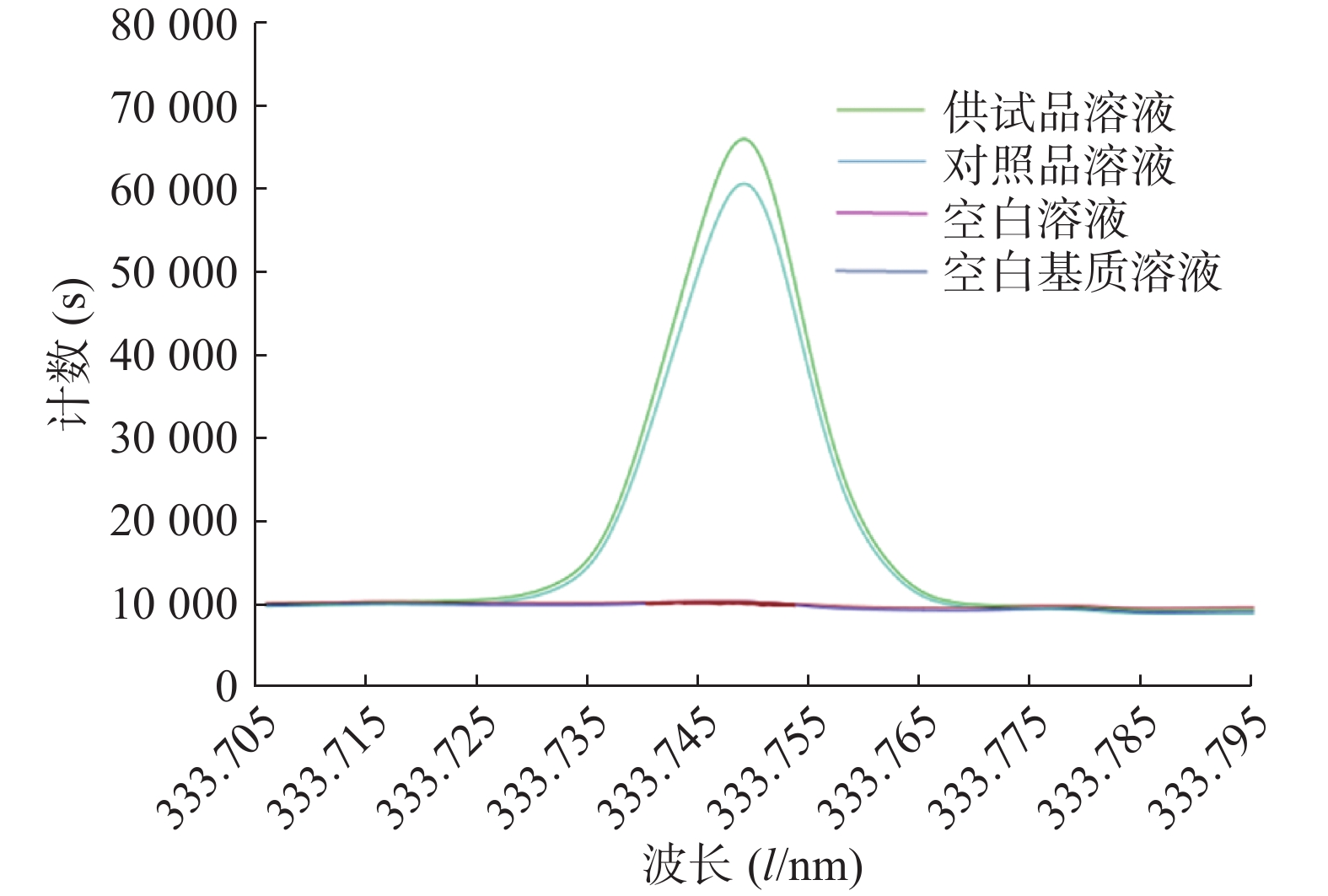
将空白溶液(2%硝酸溶液)、空白基质溶液、对照品溶液和供试品溶液依次注入等离子体光谱仪,检测并记录结果,其图谱如图1所示,结果表明La在333.749 nm处谱线干扰较少,对照品溶液和供试品溶液的峰形较好,空白溶液和空白基质溶液在此谱线无响应,方法的专属性较好。
-
将“2.4”项下制备的对照品溶液依次注入ICP-OES,检测并记录结果。结果表明,在0.1~5 μg/ml浓度范围内,仪器响应值与浓度呈良好线性关系,La的线性方程为Y=33730X-774.4,r=0.9998。
将2%硝酸溶液注入ICP-OES进行测试,连续进样11次,以空白溶液响应值标准偏差的3.3倍计算检出限,以空白溶液响应值标准偏差的10倍计算定量限。方法检出限为0.0025 μg/ml,定量限为0.0077 μg/ml。
-
精密量取空白血样9份于烧杯中,各1 ml, 分别精密加储备液0.16、0.2、0.24 ml,低、中、高每个浓度平行配制3份。随后按照“2.3”项下样品的处理方法进行制备,即得空白加标溶液。将空白基质溶液和空白加标溶液分别注入仪器,进样分析,结果见表2。La的低、中、高浓度的回收率均在94.9%~102.0%之间,表明本法的回收率良好。
加入对照品含量(μg/ml) 空白基质响应值(μg/ml) 测得值(μg/ml) 回收率(%) RSD
(%)1.6 0.0131 1.5354 95.1 0.86 1.6 0.0131 1.5610 96.7 1.6 0.0131 1.5543 96.3 2 0.0131 2.0521 102.0 1.20 2 0.0131 2.0246 100.6 2 0.0131 2.0059 99.6 2.4 0.0131 2.2914 94.9 0.85 2.4 0.0131 2.3231 96.0 2.4 0.0131 2.3272 96.4 -
取同一批血样(给药后1 h取血样本)共6份,按照“2.3”项下方法制备供试品溶液,进样分析。结果La含量的RSD为1.01%,表明本法的重复性良好。
-
4组样品在1 h的取血时点所测得的La的浓度(μg/ml)如表3所示。
编号 含量(μg/ml) RSD(%) 1 1.770±0.016 0.94 2 2.092±0.012 0.57 3 1.968±0.008 0.44 4 1.885±0.012 0.67
2.1. 全血样品采集
2.2. 仪器工作参数
2.3. 供试品溶液的制备
2.4. 对照品溶液的制备
2.5. 方法学验证
2.5.1. 专属性试验
2.5.2. 线性与定量限
2.5.3. 准确度
2.5.4. 重复性
2.6. 样品测定
-
La不是人体必需元素,已有研究表明[7, 9, 11]镧具有潜在的毒性,包括神经行为和认知行为功能障碍,可致肝功能下降,对骨、肾、脾脏、免疫系统均具有负面影响。镧可从农产品通过胃肠道进入人体,但是经皮吸收进入人体体循环的情况尚未可知,未见有La的经皮安全性评价的报道。本次试验建立了La元素的生物样本体内分析方法,为单次涂抹含镧防护乳后24 h的透皮安全性研究提供了新的方法和思路。
-
全血中无机元素的分析常通过两种途径:一是以TritonX-100水溶液或去离子水直接稀释进样测定;二是通过传统的干灰化法、湿法消解或微波消解法将血样完全消解后进样测定[12]。由于全血中含有大量蛋白质等物质,直接稀释干扰较多,与湿法、干法消解法相比,微波消解具有加热快、消解完全、消耗试剂少、节能环保等优点[13],故本研究选择微波消解法对全血样品进行处理。常用的消解溶剂有HNO3、HF、H2O2、HClO4等[14],本实验采用HNO3,即可将血液样品消解为近无色的澄清液体(消解完全),避免了H2O2、HClO4等易爆溶剂的使用。
-
本文通过微波消解-ICP-OES法,建立了生物样本中La的测定方法,该方法操作简单,分析速度快,灵敏度高,准确度好,为多效防护乳中La的含量测定和安全性评价提供依据。







 DownLoad:
DownLoad: