-
小胶质细胞是参与防御脑缺血再灌注损伤的主要免疫细胞,在中枢神经系统修复和神经发生中发挥重要作用。在缺血性中风发生后,小胶质细胞表型往往会发生改变[1-2]。小胶质细胞经活化后会极化为促炎M1型和抗炎M2型两种表型[3-4]。抗炎型小胶质细胞能通过减轻炎症反应、清除细胞碎片和释放神经营养因子来促进大脑的恢复。然而小胶质细胞除了具有神经保护作用外,还是大脑中促炎细胞因子的主要产生者。促炎型小胶质细胞能通过启动炎症级联反应释放大量的炎性细胞因子引起炎症反应,阻碍大脑恢复过程[5-6]。
乌帕替尼(upadacitinib)是一种口服的、可逆的JAK激酶(Janus kinase)抑制剂,对JAK1有较强的选择性,已被批准用于治疗类风湿关节炎[7-9],目前临床研究正在评估几种免疫介导的炎症性疾病、克罗恩病、溃疡性结肠炎等炎症相关疾病[10-11]。有报道称小胶质细胞的极化与JAK/STAT信号通路有关[12-13],却没有对于JAK1选择性抑制剂乌帕替尼在神经系统应用方面的报道。本研究通过使用BV2小胶质细胞OGD/R模型,探讨乌帕替尼对BV2小胶质细胞极化的影响及其可能的作用机制。
HTML
-
BV2细胞和PC12细胞均购自中科院上海细胞库,培养条件:高糖型DMEM培养基,含10%胎牛血清(FBS),置37 ℃,5%CO2培养箱中。
-
细胞培养箱、酶标仪(Thermo公司);水平高速离心机(Eppendorf公司);氧糖剥夺(OGD)装置(Billups-Rothenberg公司);实时定量PCR仪(美国AB7500);Odyssey双色激光成像系统(LICOR公司)。
-
乌帕替尼(货号:AK600704,美国Ark Pharm 公司);噻唑蓝(MTT)试剂盒(塞维尔生物科技有限公司);RNA提取试剂盒、反转录试剂盒(北京全式金生物技术有限公司);SYBR Green(Thermo Fisher);IL-1β、IL-6和TNF-α的ELISA试剂盒(美国R&D公司);GAPDH、JAK1、p-JAK1、STAT6、p-STAT6抗体(CST公司)。
1.1. 细胞株
1.2. 仪器
1.3. 药物与试剂
-
细胞接种在细胞培养板中,培养24 h待细胞贴壁后,用磷酸缓冲盐溶液(PBS)将孔内培养基洗净,更换为无血清无糖的DMEM培养基,将培养板置于OGD装置中,用混合气体(95%N2+5%CO2)将装置中空气置换出来,培养板连同OGD装置一起放入37 ℃培养箱中3~4 h后取出细胞,再更换为含血清的高糖DMEM培养基继续培养24 h。
-
待测细胞每孔加入MTT试剂20 μl,37 ℃孵育4 h后,将孔内液体吸尽弃去,每孔加入150 μl 二甲基亚砜(DMSO),震荡1 min使结晶物充分溶解,用酶标仪检测492 nm处吸光度值。计算生存率:每孔的吸光度值为该孔实测吸光度值减去空白吸光度值,对照组吸光度均值定为生存率100%,每孔细胞生存率=(该孔吸光度值/对照组吸光度均值)×100%。
-
BV2细胞以3×105个/ml浓度密度接种于六孔板中,待细胞长满底部后,用200 μl的枪头垂直沿直尺过圆心迅速在底部划痕,用PBS清洗3遍将划下的细胞洗净,加入无血清无糖DMEM培养基进行OGD缺氧3 h,之后更换为无血清高糖培养基恢复氧糖继续培养24 h,在恢复氧糖期间0、6、12、24 h拍照观察迁移变化。划痕图片经Image J软件处理圈出划痕区域面积S,迁移率=(1‒S/S0h)×100%。
-
收集细胞培养上清,在4 ℃、14 000/min离心10 min,以去除细胞或细胞碎屑,吸取澄清的上清培养基做后续检测。按酶联免疫吸附测定(ELISA)试剂盒(美国R&D公司)说明书进行操作,根据标准品吸光度值制作标准曲线,计算出每个样品吸光度值对应的炎症因子含量。
-
将BV2小胶质细胞正常培养或 OGD 4 h/R24 h后的培养上清液(CM)用原培养基稀释1倍后分别加入正常或者OGD 4 h处理的PC12神经细胞中,复氧共培养24 h后采用 MTT 检测PC12细胞的生存率。
-
弃去培养基,用PBS漂洗一次,六孔板中每孔加入200 μl的异硫氰酸胍裂解液(TransZol Up),反复吹打使细胞充分裂解,室温静置5 min。按全式金RNA提取试剂盒操作步骤提取RNA,之后按全式金反转录试剂盒说明书将RNA反转录成cDNA,储存于‒20 ℃用于后续实验。反转录得到的cDNA使用 7500型实时 PCR 仪进行定量分析,本实验使用内参基因 GAPDH 对模板进行均一化处理。对照组、OGD组和乌帕替尼组基因之间表达差异通过2−ΔΔCt计算。
-
弃去培养基,用预冷的PBS清洗2遍,每孔加入50 μl预冷的RIPA裂解液,放冰上裂解5 min,收集裂解液,于4 ℃、14 000 g离心15 min,收集上清。二喹啉甲酸(BCA)试剂盒测定蛋白浓度,用8% SDS-PAGE胶电泳分离蛋白,湿转至硝酸纤维素膜上,于封闭液室温封闭2 h后,更换为一抗在4 ℃孵育过夜,用等渗缓冲液(TBST)漂洗5 min(3次),加入荧光二抗室温避光孵育1 h,漂洗干净后用Odyssey双色红外激光扫描成像系统对膜进行扫描。图像条带通过image J软件进行分析,计算蛋白表达量。
-
使用SPSS 17.0软件进行统计分析,数据用均数±标准差(
$\bar x \pm s$ )表示。采用单因素方差分析检验差异的显著性,P<0.05表示有统计学意义。
2.1. OGD/R模型的建立
2.2. MTT法测细胞生存率
2.3. 细胞划痕实验
2.4. ELISA检测炎症因子
2.5. 条件培养基共培养体系的建立
2.6. 实时PCR检测mRNA水平
2.7. Western blot检测蛋白水平
2.8. 统计学方法
-
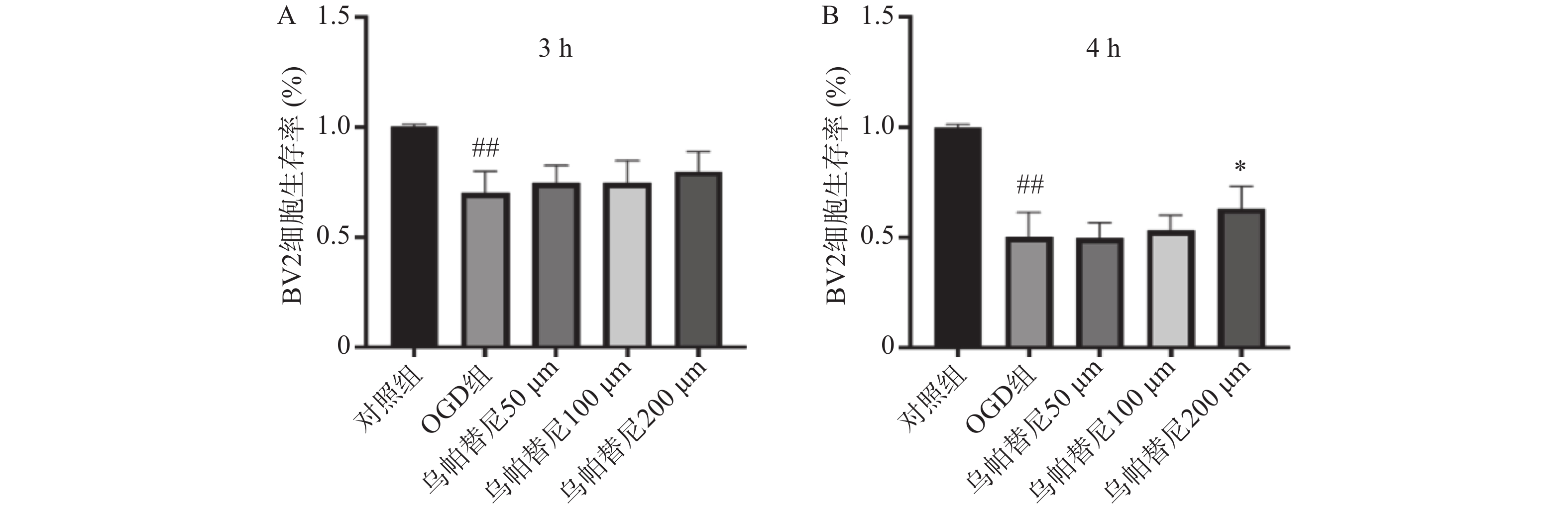
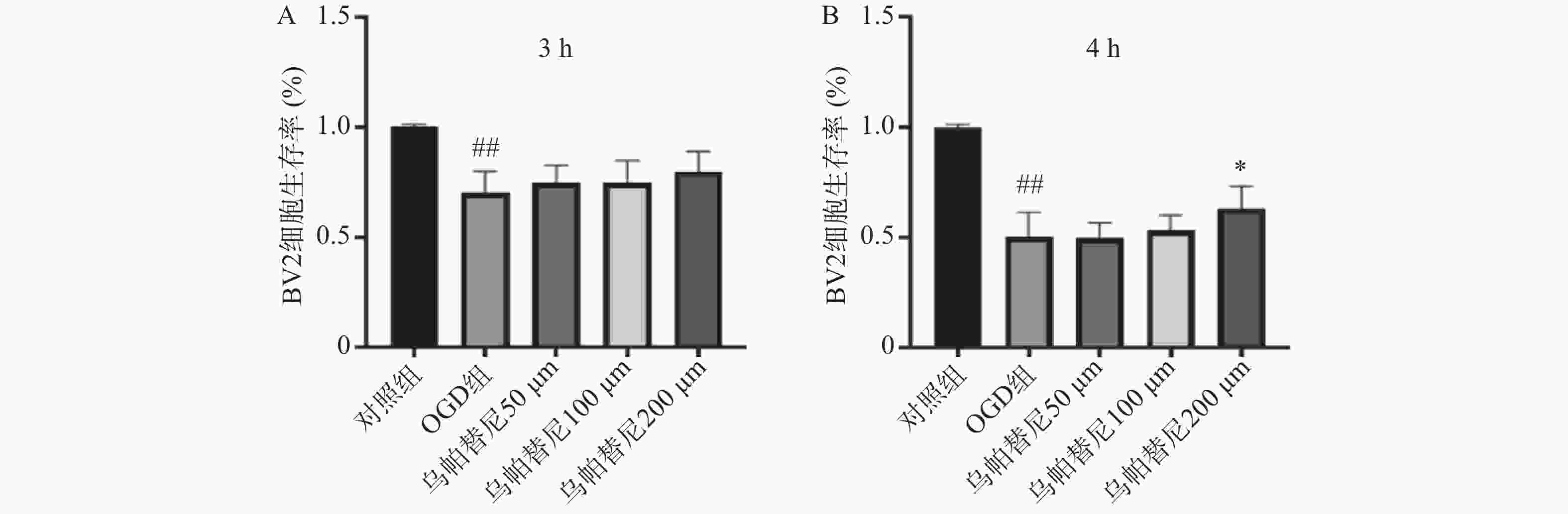
结果如图1所示,BV2细胞OGD 3 h后细胞的生存率较未进行OGD的对照组明显降低(P<0.01),给予乌帕替尼后生存率较OGD组差异无统计学意义(图1A);在OGD 4 h后,与对照组相比,OGD组细胞生存率明显降低(P<0.01),而与OGD组相比,乌帕替尼200 μmol/L浓度下能显著提高BV2细胞生存率(P<0.05)(图1B)。因此,选择OGD 4 h作为后续实验条件,选择乌帕替尼200 μmol/L作为后续实验药物浓度。
-
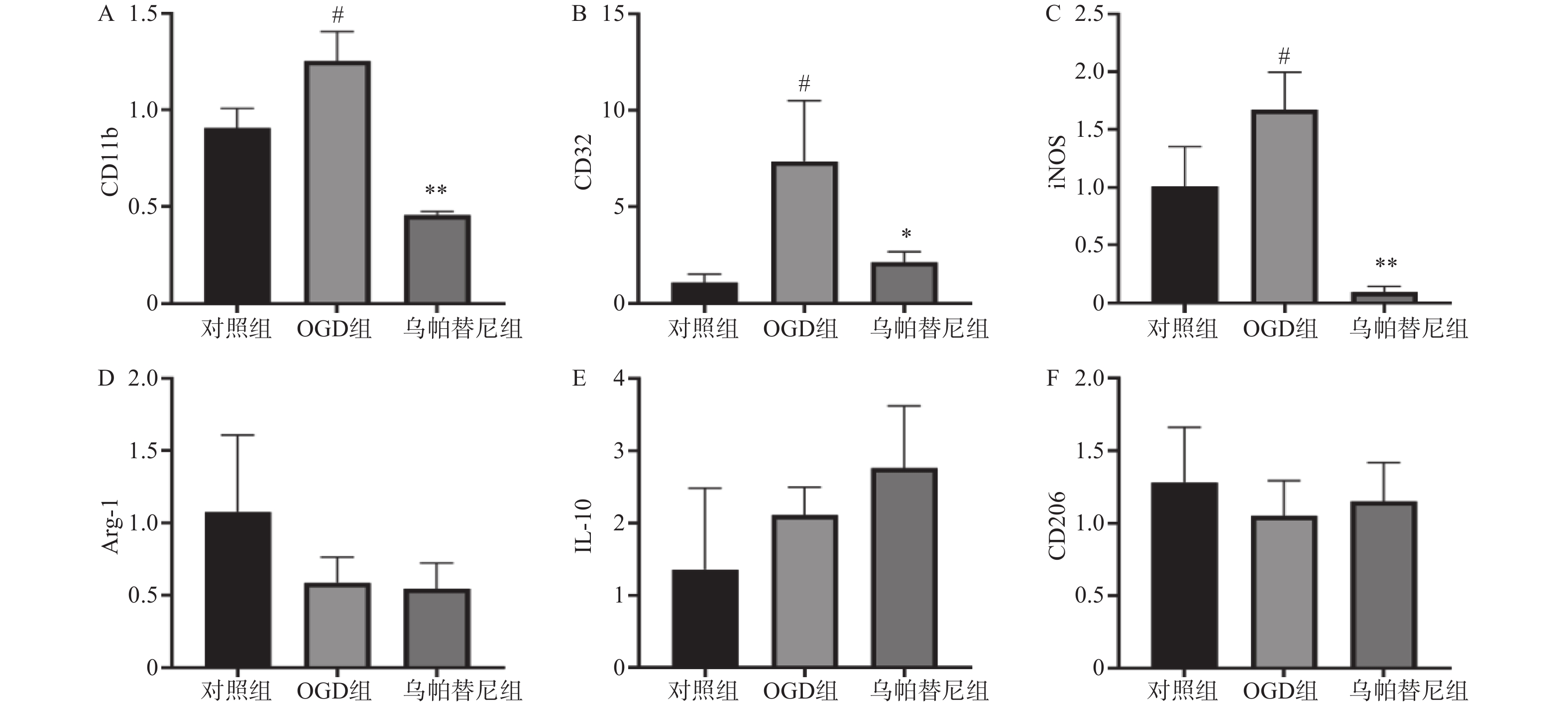
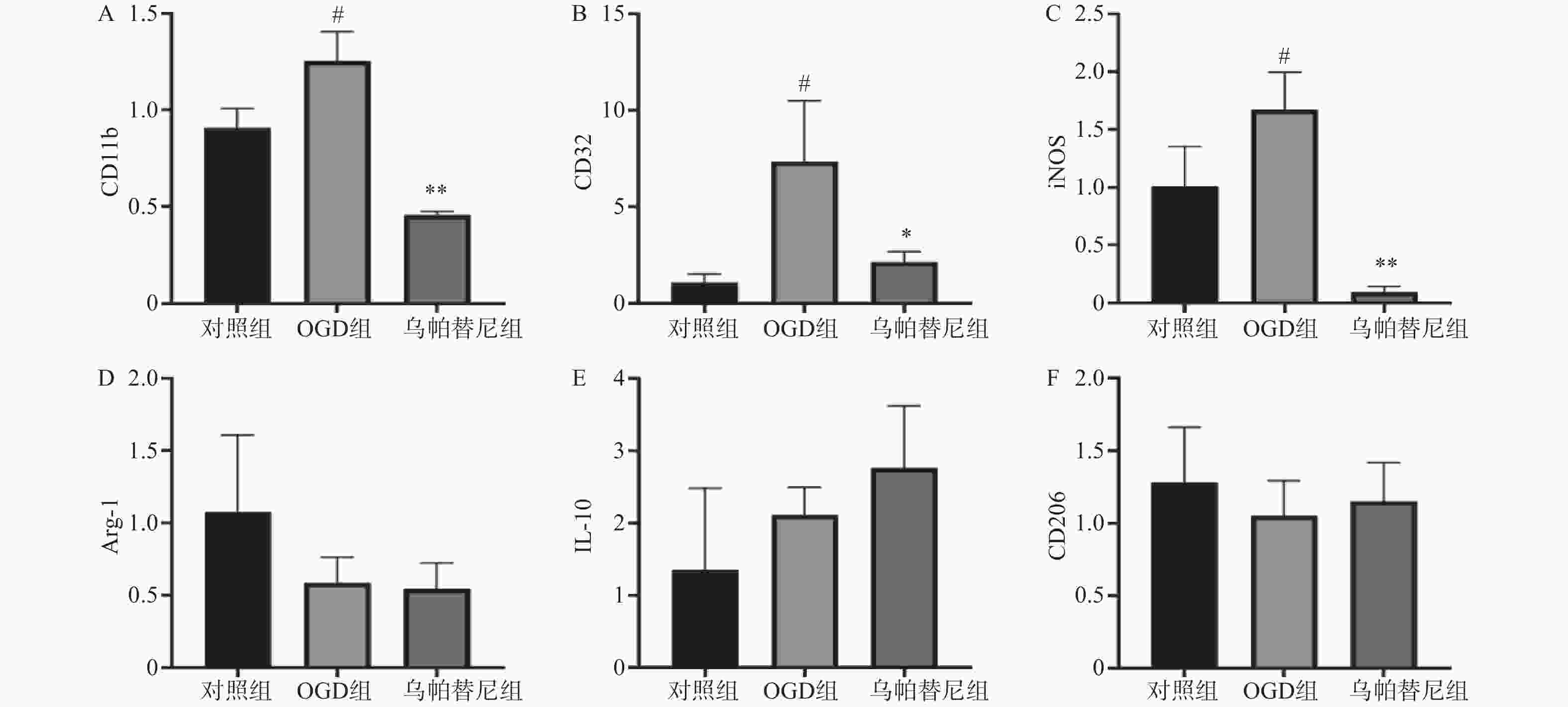
结果如图(图2)所示,与对照组相比,OGD组BV2细胞M1型标志物:CD11b、CD32、iNOS的mRNA水平均明显增加(P<0.05),而M2型标志物:Arg-1、IL-10、CD206的mRNA水平改变无统计学意义。与OGD组相比,给予乌帕替尼后CD11b(P<0.01)、CD32(P<0.05)、iNOS(P<0.01)的mRNA水平均显著减少,M2型标志物mRNA水平改变无统计学意义。
-
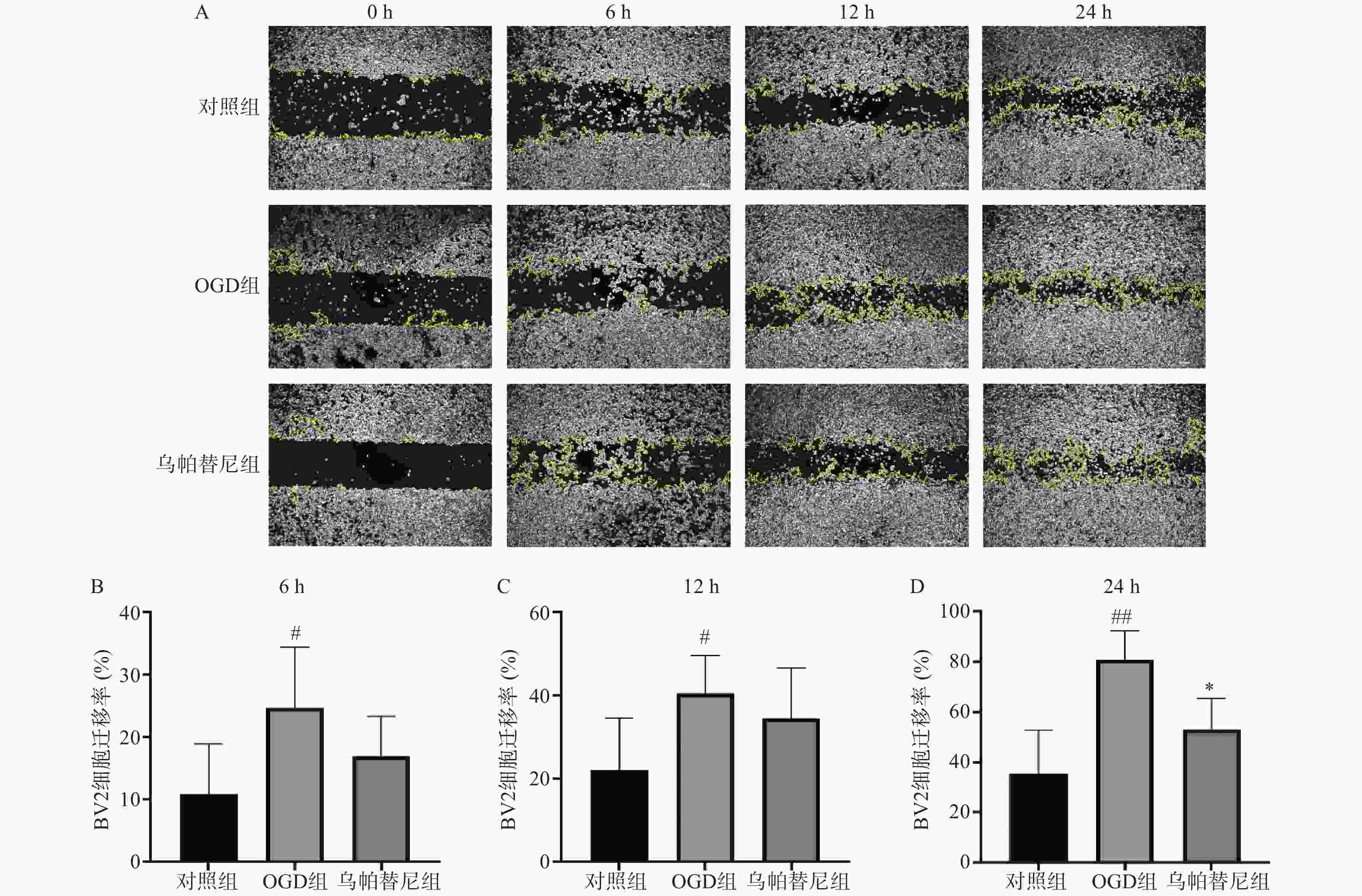
从细胞划痕实验结果(图3)中可以看出,与对照组相比,OGD组BV2细胞在6 h(P<0.05)、12 h(P<0.05)和24 h(P<0.01)细胞迁移率均明显增加,而与OGD组相比,乌帕替尼给药组在6 h和12 h细胞迁移率的改变无统计学意义,在给药24 h后的细胞迁移率明显降低(P<0.05)。
-
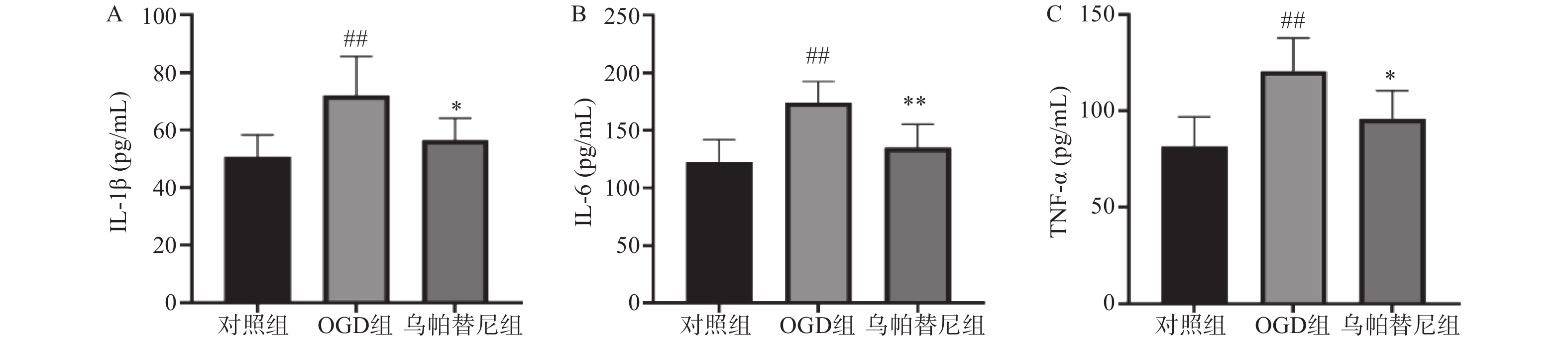
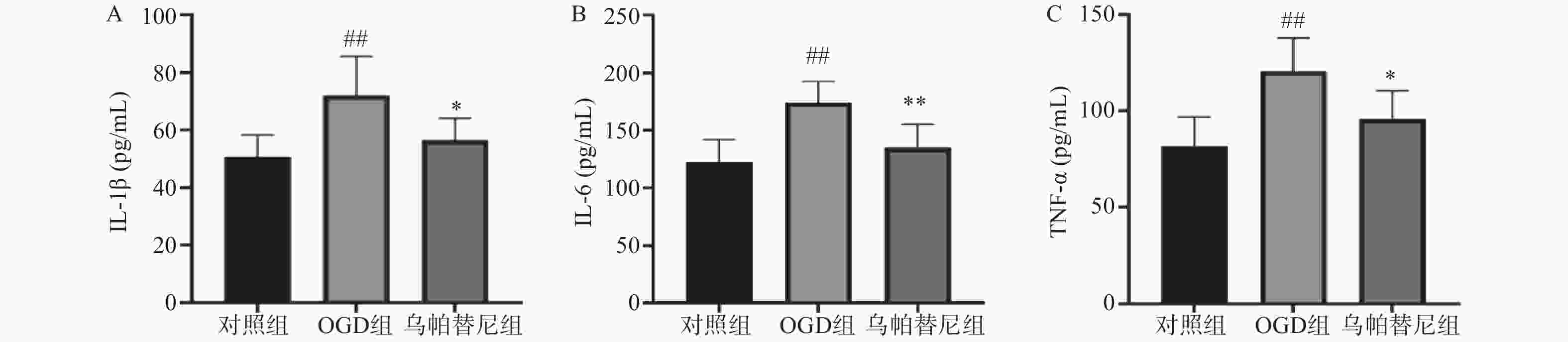
与对照组相比,OGD组培养基中IL-1β、IL-6和TNF-α水平均明显升高(P<0.01)。与OGD组相比,乌帕替尼给药组IL-1β(P<0.05)、IL-6(P<0.01)、TNF-α(P<0.05)等3种炎性因子水平均显著降低(图4)。
-
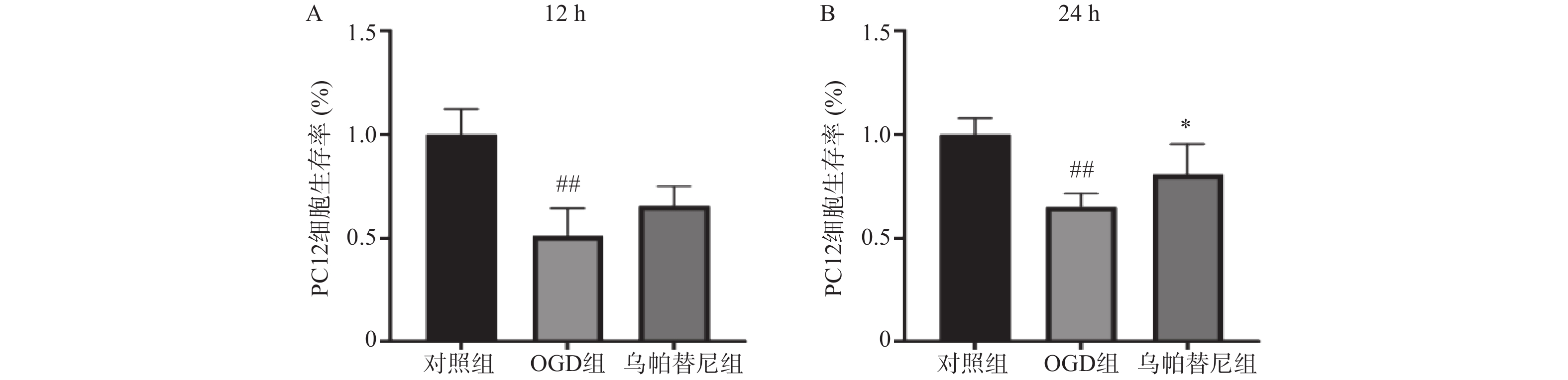
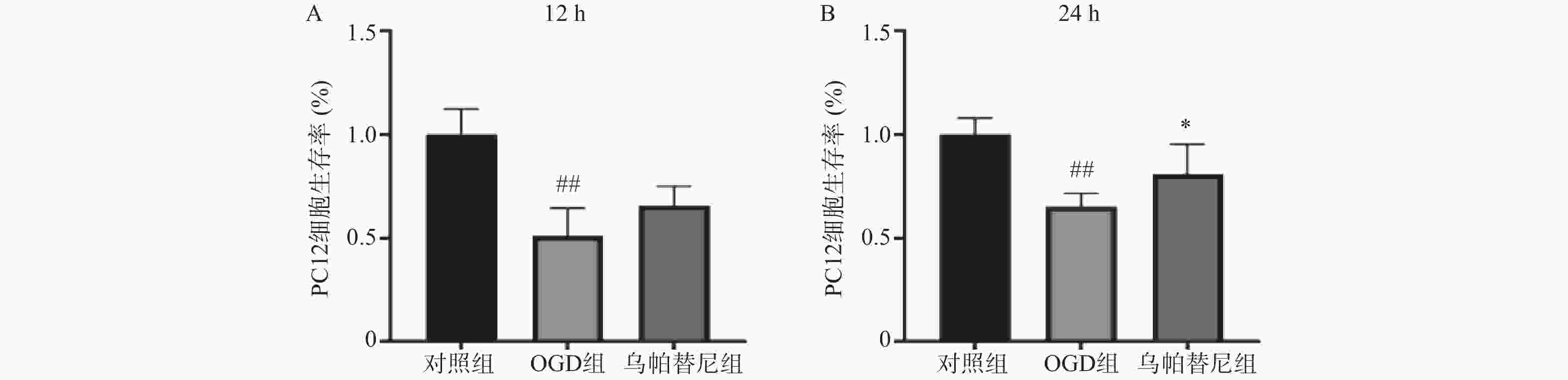
结果如图所示,与对照组相比,条件培养基共培养12 h的OGD组PC12细胞生存率明显降低(P<0.01),在给予乌帕替尼条件培养基后,PC12细胞生存率较OGD组增加无统计学意义(图5A);共培养24 h的OGD组细胞生存率较对照组明显减少(P<0.01),而乌帕替尼组较OGD组PC12细胞生存率明显增加(P<0.05)(图5B)。
-
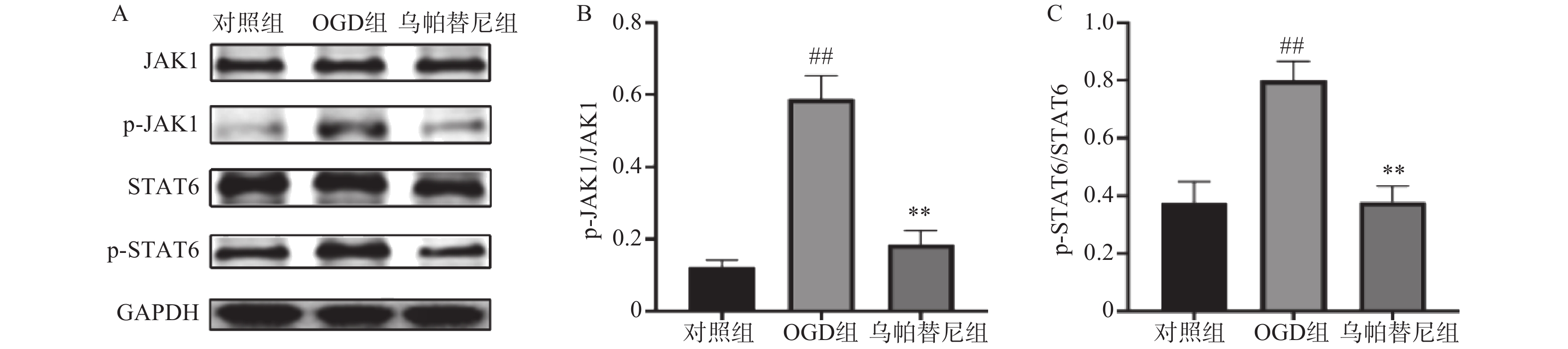
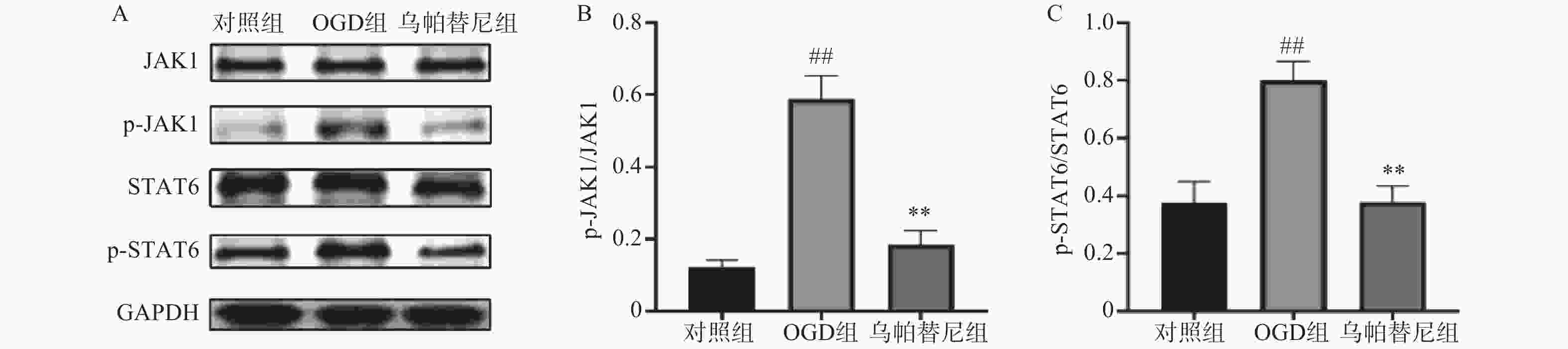
结果如图所示,在OGD/R损伤后,BV2细胞p-JAK1和p-STAT6蛋白表达水平较对照组显著增加(P<0.01),在给予乌帕替尼后,p-JAK1和p-STAT6蛋白表达水平较OGD组显著降低(P<0.01)(图6B, 6C)。
3.1. 乌帕替尼对OGD/R诱导的BV2细胞损伤的影响
3.2. 乌帕替尼对OGD/R后BV2细胞极化的影响
3.3. 乌帕替尼对BV2细胞OGD后迁移能力的影响
3.4. 乌帕替尼对BV2细胞OGD/R后培养基中炎性因子水平的影响
3.5. 乌帕替尼对共培养PC12细胞OGD后细胞生存率的影响
3.6. 乌帕替尼对OGD/R后BV2细胞JAK1/STAT6信号通路相关蛋白表达水平的影响
-
神经炎症与神经系统疾病密切相关[14],而小胶质细胞是神经系统参与炎症反应的关键细胞,小胶质细胞经刺激后活化,根据其激活状态分为M1型和M2型2种表型,中枢神经系统(CNS)中的小胶质细胞可以是促炎的,也可以有神经保护的作用,这取决于它们的激活状态。促炎型小胶质细胞表达促炎因子IL-1、TNF-α、IL-6、一氧化氮(NO)等,会对神经系统有不利影响[15];而抗炎型小胶质细胞则会引起多种抗炎因子释放,如FIZZ1、Chi3I3、精氨酸酶1、Ym1、CD206、胰岛素样生长因子1(IGF-1)和Fzd1等,这些细胞因子可能参与神经保护和组织愈合[16]。
哺乳动物JAK激酶家族由3个JAKs(JAK1、JAK2、JAK3)和酪氨酸激酶2(TYK2)组成,它们经多种细胞因子触发,选择性地结合不同的受体,从而作为信号传导器在细胞内发挥作用[17]。JAK激酶激活下游的STAT蛋白,激活的STAT蛋白作为转录因子,转运到细胞核,上调促炎性细胞因子和生长因子基因[18]。本研究将JAK1选择性抑制剂乌帕替尼作用于OGD/R后的BV2小胶质细胞,结果显示乌帕替尼能增加OGD/R后BV2细胞的生存率。在发生急性脑卒中后,脑内的小胶质细胞通常会迁移至损伤部位[19],而受到损伤部位刺激后,小胶质细胞会极化为M1型加重了缺血损伤[20],于是笔者对BV2小胶质细胞OGD/R后的极化和迁移能力进行检测,发现BV2细胞在OGD/R后,其M1型标志物CD11b、CD32、和iNOS的mRNA水平明显升高,在乌帕替尼处理后,M1型标志物水平明显减少,这个结果表明,乌帕替尼能抑制BV2小胶质细胞向M1型极化,划痕实验结果可以看出乌帕替尼能抑制BV2细胞OGD/R后的迁移能力。为了进一步研究小胶质细胞极化后对神经系统的影响,本实验检测了OGD/R后BV2细胞培养基中炎症因子的含量,研究结果显示,乌帕替尼可减少OGD/R后BV2细胞分泌的炎症因子。之后将OGD/R后的BV2细胞条件培养基稀释一半后加入到OGD 4 h后的PC12细胞中,实现OGD/R后BV2细胞的条件培养基与OGD后PC12细胞之间的共培养,共培养结果显示,经乌帕替尼处理的BV2细胞的条件培养基能提高OGD/R后PC12细胞的生存率。乌帕替尼为JAK1选择性抑制剂,而JAK/STAT信号通路与神经炎症密切相关,于是本研究通过Western blot检测乌帕替尼对OGD 4 h/R 24 h后BV2细胞JAK1/STAT6信号通路相关蛋白的表达水平,发现经OGD/R处理后的BV2小胶质细胞JAK1和STAT6磷酸化蛋白的表达水平明显增加,而乌帕替尼处理后可明显减少JAK1和STAT6蛋白的激活,从而抑制BV2细胞炎症通路,减少由BV2小胶质细胞经激活后引起的神经炎症。
综上所述,乌帕替尼能抑制BV2细胞经OGD/R刺激后向M1型极化,抑制其迁移能力并减少炎症因子的释放,通过调控JAK1/STAT6信号通路抑制炎症反应起到神经保护的作用。乌帕替尼目前已被批准用于类风湿关节炎的治疗,但是对神经系统炎症疾病治疗的研究非常少,其抑制JAK/STAT信号通路对脑缺血损伤可能具有保护作用。








 DownLoad:
DownLoad: