-
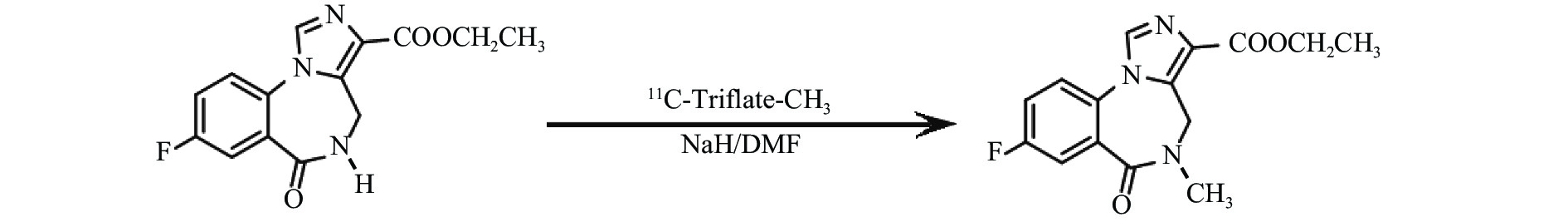
氟马西尼(flumazenil,FMZ)作为中枢苯二氮䓬受体(CBZR)拮抗剂,具有高度特异性和选择性,通过拮抗苯二氮䓬类药物与其受体的结合,用于逆转其所致的中枢镇静作用[1]。11C-氟马西尼(11C-flumazenil,11C-FMZ)可通过PET/CT显像定量分析CBZR与γ氨基丁酸(GABA)受体、氯离子通道偶联形成的复合受体GABA/CBZR,用于癫痫病灶及遗传性舞蹈病的检查[2]。但是11C的半衰期只有20.38 min,制备结束后如不及时注射入受检者体内,每摩尔(mol)药液的放射性活度,即比活度会很快降低。11C-FMZ在体内经肝酶水解,部分转变为无CBZR拮抗活性的羧酸代谢物[3],见图1。不同比活度的11C-FMZ在体内代谢有无差异,未见文献报告。本研究通过测定不同比活度的11C-FMZ在体内的代谢变化,拟为11C-FMZ的临床合理应用提供一定依据。
HTML
-
Minitrace回旋加速器、TRACELab FXc放射性药物合成系统(美国GE公司);HPLC半制备分析系统、紫外检测器、Sep-Pak C18层析柱(美国Waters公司);LB508型放射性检测器(德国Berthold公司);CRC-15R型活度检测器(美国Capintec公司);Millex-GS无菌滤膜(美国Capintec公司);Wallac 1470 WIZARD γ计数器(芬兰PE公司),WD-9405B型水平摇床(北京沃德生物医学仪器公司),高速低温离心机(美国Therma公司)。
去甲基氟马西尼、标准品氟马西尼(江苏华益科技有限公司);碘(美国Sigma-Aldrich公司);氢气、氦气、氮气、1%氧-氮混合气、盐酸、磷酸、无水乙醇、无水乙腈、二甲基甲酰胺(DMF)、氢化钠(NaH)、0.9%氯化钠(中国医药集团上海化学试剂公司);三氟磺酸银粉(Ag-Triflate,自制)。商品化溶剂皆为市售分析纯或化学纯。
-
选择2019年5月至10月北部战区总医院核医学科10名健康受试者,其中,男性5名,女性5名,平均年龄(41.7±4.7)岁,平均体重(69.3±6.8)kg。所有受试者均进行系统的体格检查,确认无基础疾病且试验前1个月内无药物服用史,并签署了知情同意书。
-
首先使用回旋加速器以1% O2/N2为原料,通过14N(p,α)11C核反应生产11C-CO2,传输至药物合成系统,并以图2所示,合成路线转化为11C-三氟甲基磺酰基甲烷(11C-Triflate-CH3)[4]。将0.5 mg去甲基氟马西尼和1 mg NaH溶于0.4 ml DMF于反应池中与11C-Triflate-CH3在0 ℃反应5 min,升至室温并加入0.5 mol/L HCl中和。混合物进入HPLC进行分离纯化,分离柱:Nucleosil 100-5 C18,250 mm×10 mm,流动相:含0.01 mol/L磷酸的22%乙腈溶液,检测波长254 nm,流速4 ml/min。收集放射性峰组分,用Sep-Pak C18柱再次分离纯化,最后通过0.22 μm的无菌滤膜过滤得到产物11C-FMZ[5],如图3所示。产品依据《中国药典》(2015年版)进行无菌检测和内毒素含量检测。
-
首先,在静息状态下通过肘静脉为10名受试者注射11C-FMZ 3 ml,经活度检测器测量,平均放射性活度为(10.5±2.9)mCi(1 Ci=3.7×1010 Bq),放射性比活度以单位物质的量所含放射性活度作为定量依据,平均比活度为(268.3±57.2)×103Ci/mol;2 h后,再次注射11C-FMZ 3 ml,平均活度为(10.6±2.8)mCi,此时药液已放置5~6个半衰期,平均比活度降为(57.8±11.4)×103Ci/mol。两次注射药物后均需静卧60 min,分别在1、2、3、5、10、15、20、30、40、60 min时于对侧肘静脉抽取血样。
-
取500 μl血样于试管中,加入500 μl缓冲溶液(0.1 mol/L硼酸,0.05 mol/L氯化钾,0.1 mol/L氢氧化钠)调节pH至11,再加入1 ml氯仿,于水平摇床混匀5 min,再以4 000 r/min离心5 min。取氯仿相和水相各200 μl,使用γ计数器分别测定两相1 min的放射性计数(counts per minute,cpm)。
-
放射性计数首先需经衰变公式(公式1)校正以抵消血浆预处理的耗时所致的放射性衰减,再计算各血样的百分注射剂量率(%ID/L,公式2)及不同比活度下各时间点11C-FMZ羧酸代谢物的占比(公式3)。使用SPSS 18.0软件对数据进行统计学分析,符合正态分布的计量资料以(
$ \stackrel{-}{x}\pm s $ )表示,采用配对样本均数t检验,P<0.05为差异有统计学意义。I0:抽血时的cpm,I:测量时的cpm,t:抽血与测量的时间差 (min),T:11C半衰期20.38 min。
首先计算t/T值,从“衰变计算表”[6]中查出比值对应的e-λt值。
1.1. 仪器与试剂
1.2. 研究对象
1.3. 研究方法
1.3.1. 11C-FMZ的合成
1.3.2. 药物的注射
1.3.3. 血浆预处理与放射性计数测定
1.4. 计算与统计学分析
-
11C-FMZ的百分注射剂量率随时间呈指数下降,羧酸代谢物则先升后降并在10 min后开始超过11C-FMZ,如表1所示。羧酸代谢物的百分占比随时间延长而逐步增加,于15 min后基本趋于稳定,且低比活度组的百分占比明显高于高比活度组,从统计学结果可以看到,两组数据在15~60 min的区间存在显著统计学差异(P<0.05),如表2所示。
测定成分 时间(t/min) 1 2 3 4 5 10 15 20 30 40 60 11C-FMZ① 9.76±4.80 6.00±1.42 3.30±0.60 2.77±0.39 2.25±0.33 1.95±0.24 1.55±0.15 1.28±0.18 1.09±0.14 0.98±0.15 0.78±0.10 羧酸代谢物① 0.04±0.04 0.19±0.10 0.44±0.13 0.51±0.14 0.67±0.13 1.88±0.24 2.00±0.33 1.73±0.28 1.50±0.28 1.59±0.20 1.22±0.22 11C-FMZ② 13.84±4.67 6.10±1.31 3.27±0.49 3.10±0.36 2.66±0.37 1.97±0.33 1.42±0.13 1.23±0.15 1.11±0.06 0.67±0.13 0.45±0.07 羧酸代谢物② 0.15±0.11 0.28±0.08 0.50±0.17 0.55±0.29 0.98±0.27 2.22±0.42 2.36±0.44 2.39±0.34 1.90±0.41 1.39±0.25 0.98±0.14 注:①表示高比活度(268.3±57.2)×103 Ci/mol注射后的剂量率;②表示低比活度(57.8±11.4)×103Ci/mol注射后的剂量率。 11C-FMZ羧酸代谢物 时间(t/min) 1 2 3 4 5 10 15 20 30 40 60 高比活度 0.54±0.54 3.32±1.89 12.04±3.61 15.58±4.25 23.11±4.92 48.98±4.89 56.11±4.68 57.36±5.63 57.60±3.45 61.92±4.51 60.85±5.39 低比活度 1.12±0.70 4.53±1.69 13.48±4.94 14.64±6.01 27.04±7.81 52.90±5.81 62.04±4.69* 65.85±5.35* 62.54±5.75* 67.26±4.88* 68.26±4.47* *P<0.05,与高比活度组比较。
-
本研究使用氯仿作为11C-FMZ及其羧酸代谢物的分离试剂,对11C-FMZ的提取效率可达100%,而代谢物可完全保持在水相中。相关研究中,TLC没有检测到除羧酸代谢产物以外的其他放射性代谢物[7],保证了该方法对放射性比活度测定的准确性。
Debruyne等[8]研究了进食对11C-FMZ体内代谢的影响,并观察到进食后的代谢率更高,并认为是进食导致肝脏血流增加,从而导致肝脏提取量高的药物代谢增加。由于11C-FMZ主要通过肝脏代谢从体内清除,仅0.2%的给药剂量在尿中以原形排出[9],因此在PET研究中,应控制饮食条件。
每升血浆中所含的标记药物的百分比与之前的某些定量研究结果基本一致。Samson等[10]和Shinotoh等[11]对高放射性比活度研究的结果分别为10 min时(2.4±0.9)和(3.7±1.6)%ID/L,20 min时(2.1±0.4)和(3.5±1.4)% ID/L。但二者均缺少低放射性比活度及羧酸代谢物的进一步研究。本研究中血浆的百分注射剂量率在注射后10 min左右出现上升,与之前相关研究结果相似[11-13],这可能与标记的羧酸代谢物的产生有关。Klumpers等认为原因是药物在分布阶段暴露于酯酶的某种首过效应,这种酯酶在肝脏、肾脏和大脑中大量存在且具有非常广泛的底物特异性[14]。在狒狒身上进行的相关研究没有观察到该现象[8],这种差异是由于在该物种中羧酸代谢物的标记分数较低。本研究中的高、低比活度对应代谢物的占比分别为61%和68%,而狒狒仅为45%。
综上所述,放射性比活度不同的11C-FMZ注射后的代谢率存在明显差异,在PET相关研究中应尽量避免对比活度差异过大的同一批受试者进行试验,临床应用时同一患者在复查时所采用的比活度也需尽量与初诊时一致,以获得最佳的图像对比价值。










 DownLoad:
DownLoad: