-
保护血管内皮对维护心血管系统健康有着重要意义[1]。近年的研究证实,血管内皮完整性的维持不仅依赖于现有的内皮细胞,而且与骨髓来源的具有向成熟内皮细胞分化能力的未成熟细胞—内皮祖细胞(endothelial progenitor cell, EPC)的募集有关[2-3]。临床研究证实,外周血中循环EPC的数量与冠心病常见的危险因素(如高血压、糖尿病等)呈负相关关系,EPC可作为治疗缺血、肺动脉高压等血管疾病状态的新靶点[2-3]。本课题组和他人的研究均证实[2, 4-5],高血压动物和患者的EPC均出现显著的功能受损。因此,如果能够通过药物干预手段逆转其受损的EPC功能,无疑对减轻高血压患者与内皮功能相关的靶器官损伤,以及改善高血压EPC移植治疗效果等具有重要意义。
去氧皮质酮/盐(Deoxycorticosterone acetate-salt, DOCA-salt)高血压模型是一种盐敏感性高血压动物模型,盐敏感性高血压在美国高血压患者中占1/3的比例,是一种重要的高血压类型[4-6]。课题组既往的研究证实,DOCA-salt高血压小鼠EPC功能受损与其内皮型一氧化氮合酶(endothelial nitric oxide synthase, eNOS)的脱偶联(uncoupling)有关[5]。在研究中我们也找到了一些对eNOS脱偶联相关的信号通路/分子有一定改善作用的药物,如四氢生物蝶呤(tetrahydrobiopterin, BH4),超氧化物歧化酶-聚乙二醇(superoxide dismutase-polyethylene glycol, PEG-SOD),N(G)-硝基-L-精氨酸(N(G)-nitro-L-arginine, L-NNA)等[5-6]。但目前尚不知道这些药物是否可有效逆转DOCA-salt小鼠受损的EPC功能。本研究拟在以往研究的基础上,寻找改善盐敏感性高血压小鼠EPC功能失常的有效药物干预手段,为进一步的EPC移植治疗研究奠定基础。
-
野生型(C57BL/6)雄性小鼠,10~12周龄,体重20~25 g(上海斯莱克实验动物中心)。
-
BH4、PEG-SOD、L-NNA(美国Sigma公司),EGM-2培养基(Lonza公司),胎牛血清(美国GIBCO公司),Matrigel胶(美国BD公司)。
-
选用10~12周龄,体重20~25 g野生型(C57BL/6)雄性小鼠进行模型制作。小鼠麻醉,侧卧位固定,腹部纵行切口,暴露左肾,小心结扎肾动脉和肾静脉后,进行左肾切除,缝合切口。然后在小鼠两前臂中间的位置开一个大约1 cm的纵行切口,皮下埋置150 mg/kg的DOCA缓释片。DOCA组小鼠给予含1.0% NaCl和0.2% KCl的盐水。假手术组小鼠也进行左肾切除,但不给DOCA缓释片,而且饮用常规自来水。手术后,所有动物被单独饲养于一个干净的塑料笼内。术后第21天用尾动脉测压法测定动物的收缩压水平。
-
将玻璃粘连蛋白(vitronectin)加入6孔板预处理后备用。小鼠麻醉后处死。使用无菌器械剥离小鼠双侧后肢股骨、胫骨并剔除肌肉组织,于超净台内用EGM-2培养基冲出骨髓。将骨髓细胞浓度调定至2.5×105 /cm2后,加入预处理的6孔板内培养(37 ℃、5% CO2)。第4天弃去原培养基,更换新鲜培养基,第7天使用。
-
本研究采用了以下两种鉴定方法。
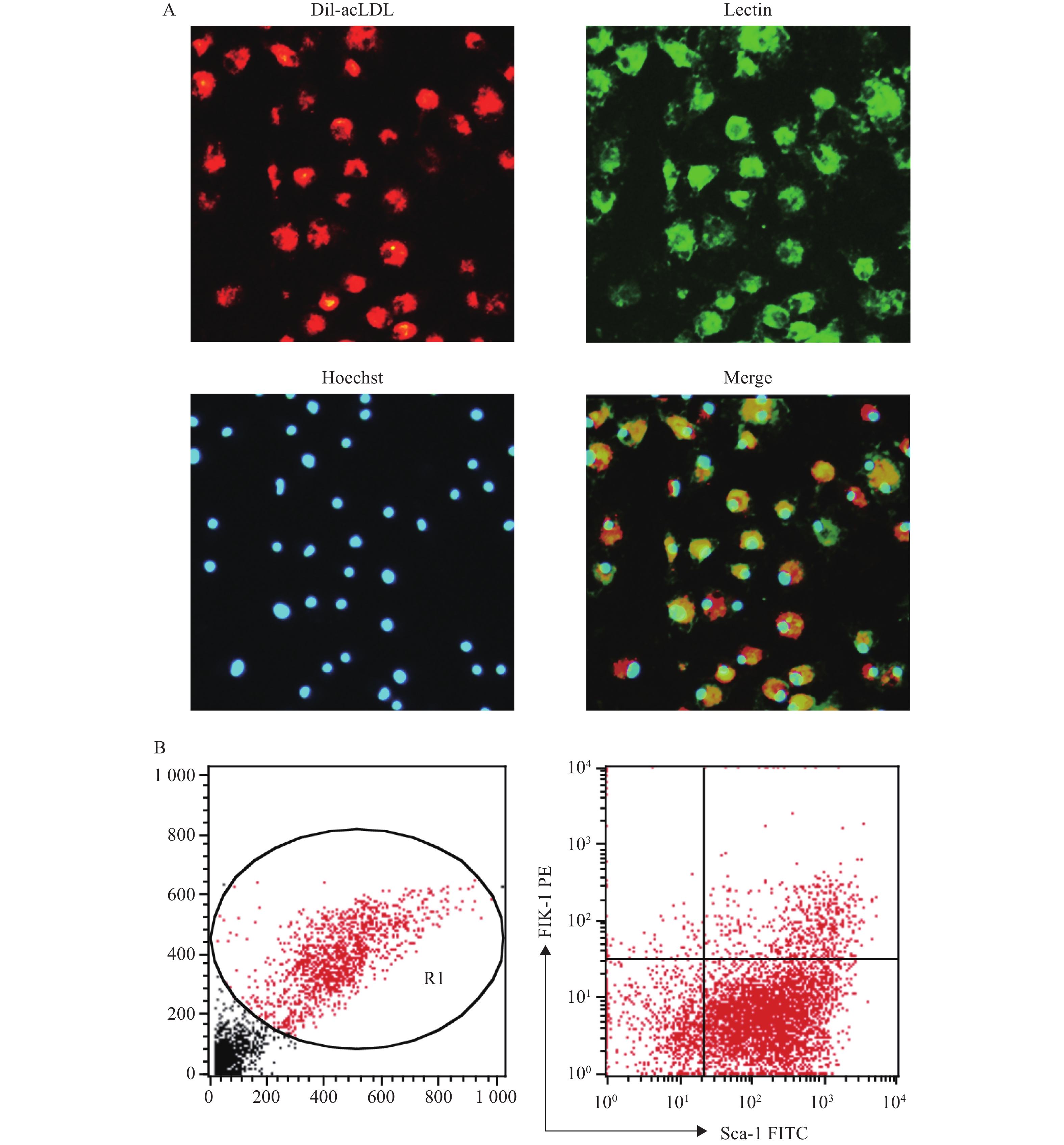
(1)流式细胞仪法:EPC培养至第7天后,用0.125%胰酶-0.02% EDTA消化,洗涤后调整细胞密度为2×106 /ml,将细胞与Sca-1的抗体(1 µg/ml)和Flk-1的抗体(1 µg/ml)在冰上共孵育1 h,注意避光。之后用5%BSA/PBS洗涤,再用2%的多聚甲醛固定10 min,用流式细胞仪检测Sca-1、Flk-1双标阳性的细胞,即为EPC。
(2)荧光显微镜法:EPC培养至第7天后,用PBS洗细胞,加入1 mg/ml Dil-acLDL(1:200),于37 ℃避光孵育4 h,用PBS洗细胞,加2%多聚甲醛固定10 min。加10 µg/ml的Lectin,室温避光孵育1 h,再用PBS洗细胞。加Hoechst 33258(10 µg/ml),室温避光孵育30 min,用PBS洗细胞,加2%多聚甲醛固定10 min后,在荧光显微镜下观察Dil-acLDL(红色荧光)、Lectin(绿色荧光)、Hoechst 33258(蓝色荧光)3种染料染色阳性的细胞,即为EPC。
-
小鼠骨髓来源EPC培养至第6天后换液,分别用BH4 (10 µmol/L), PEG-SOD (100 U/L)和L-NNA (0.8 mmol/L)3种药物孵育24 h后,测定其小管形成功能和黏附功能。
-
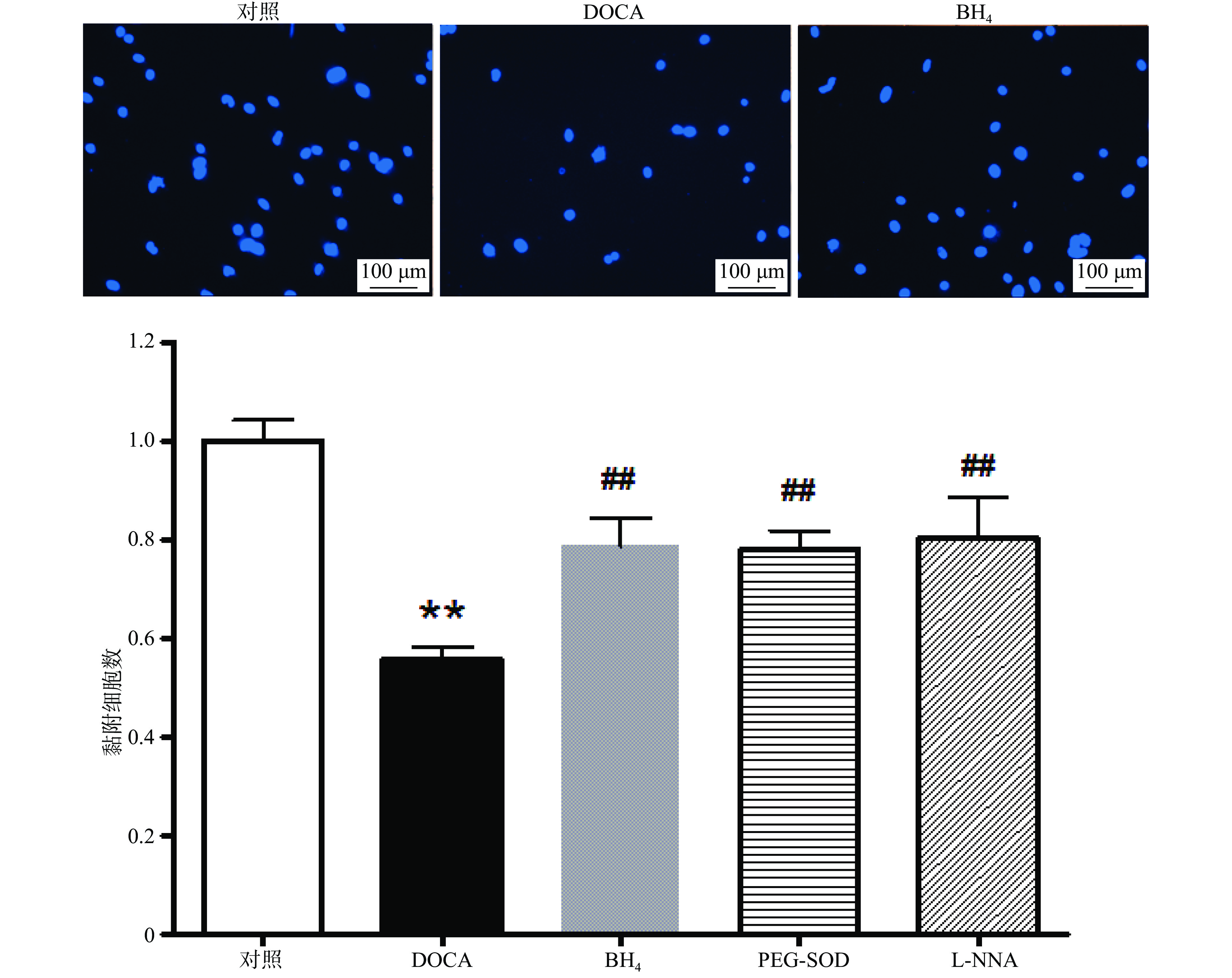
培养7 d的EPC用0.125%胰酶−0.02% EDTA消化,将1×104细胞接种于vitronectin包被好的96孔板中,每个样本设4复孔。37 ℃、5% CO2孵育24 h后,去除培养基,PBS洗涤3次,然后用Hoechst 33258 (5 µg/ml)染细胞核,再用PBS洗涤3次。在荧光显微镜下,随机选取3个视野(100×)计算黏附细胞数并取平均值。
-
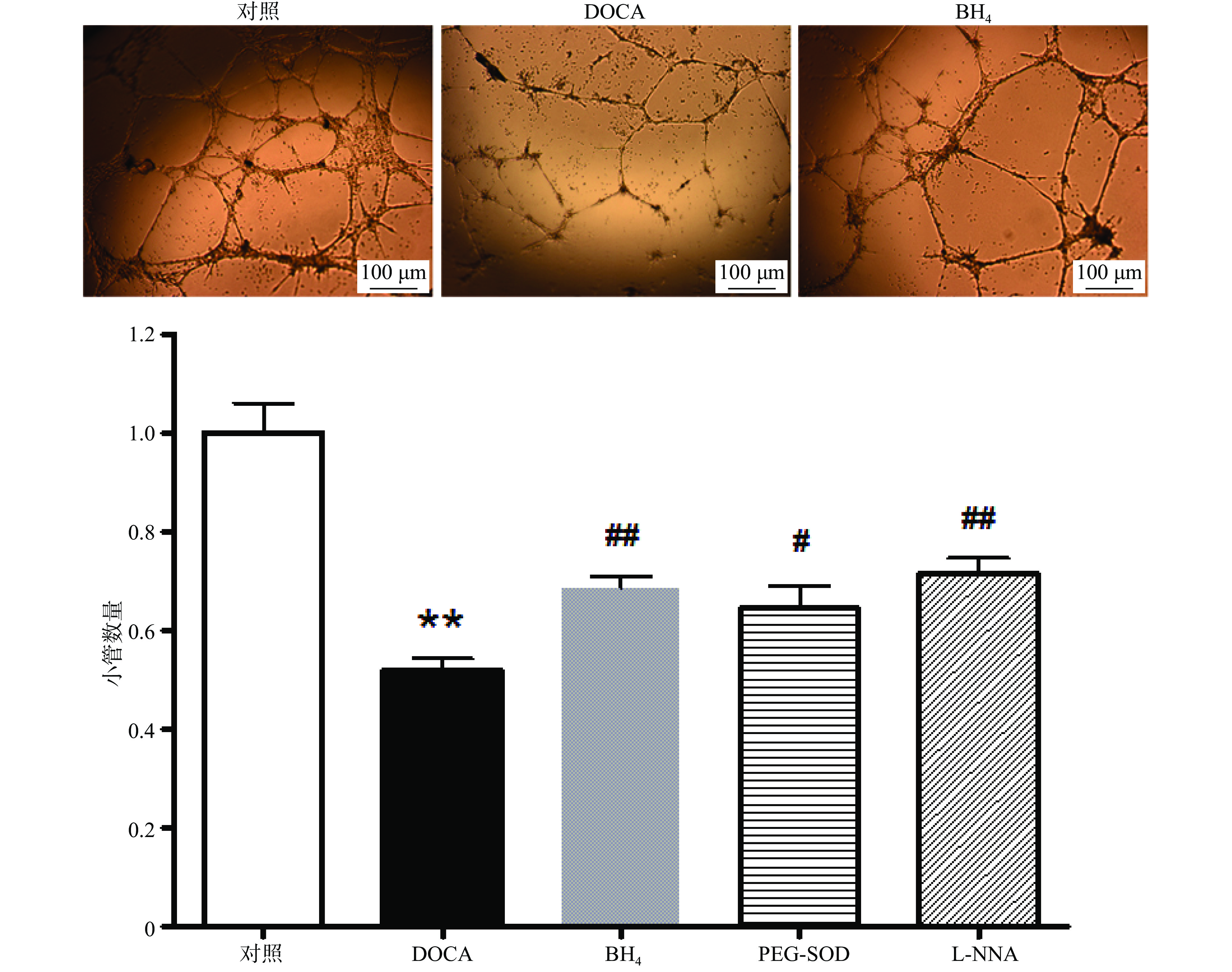
在冰上预冷的96孔板中加入60 µl的matrigel,于37 ℃孵育30 min。培养7 d的EPC用0.125%胰酶−0.02% EDTA消化,将5×104细胞接种于matrigel上,在37 ℃,5% CO2孵箱中孵育6 h后,于显微镜下随机选取4个视野(40×)计算形成小管的数目并取平均值。
-
所得实验数据均通过Prism统计软件进行分析,分别选取配对t-检验和单因素方差分析(One-Way ANOVA)作为2组数据和3组以上数据之间比较的统计学分析方法;选用Newman Keuls法进行均数之间的多重比较。数据书写格式为(均数±标准误)。以P<0.05表示差异有统计学意义。
-
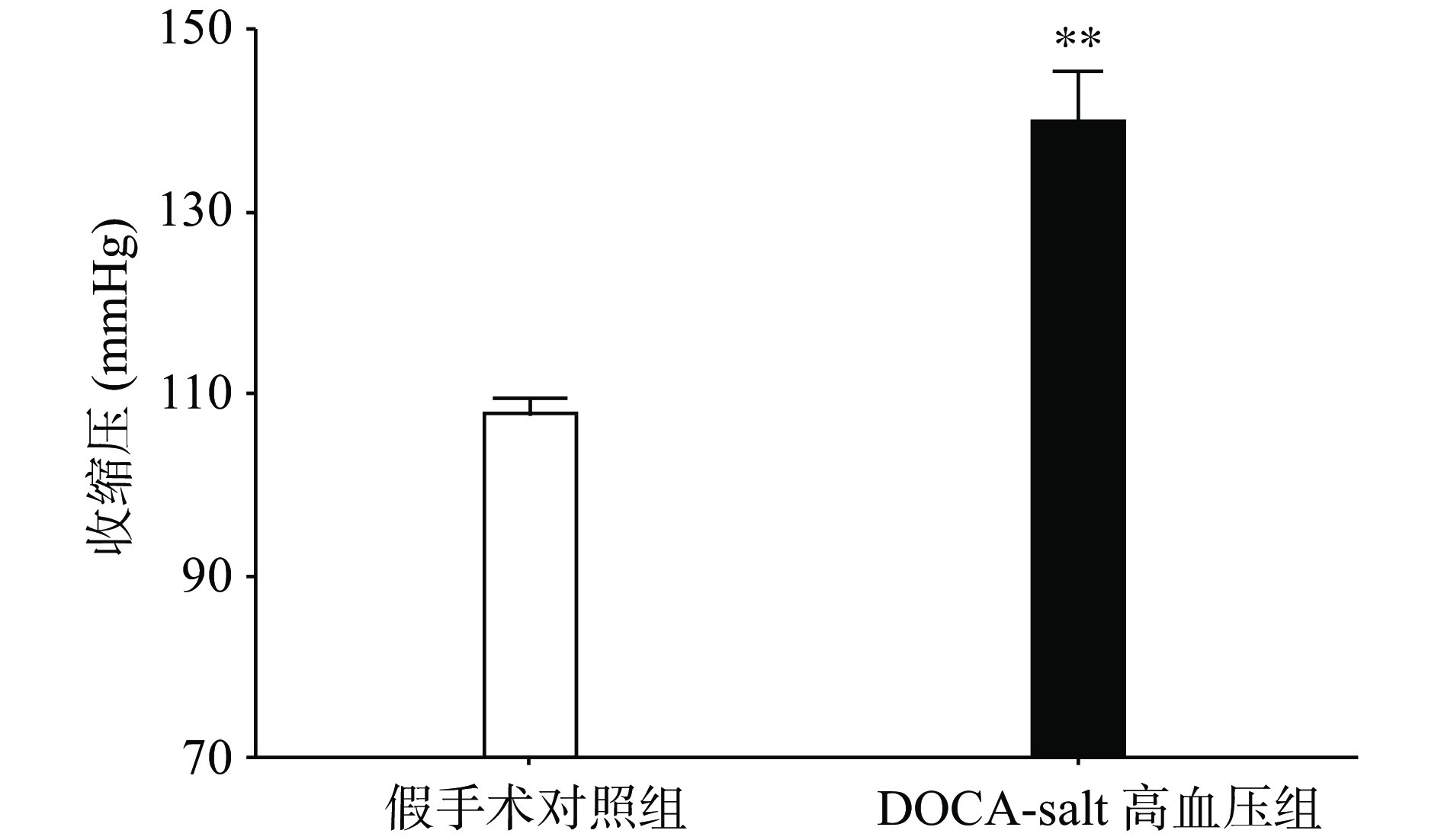
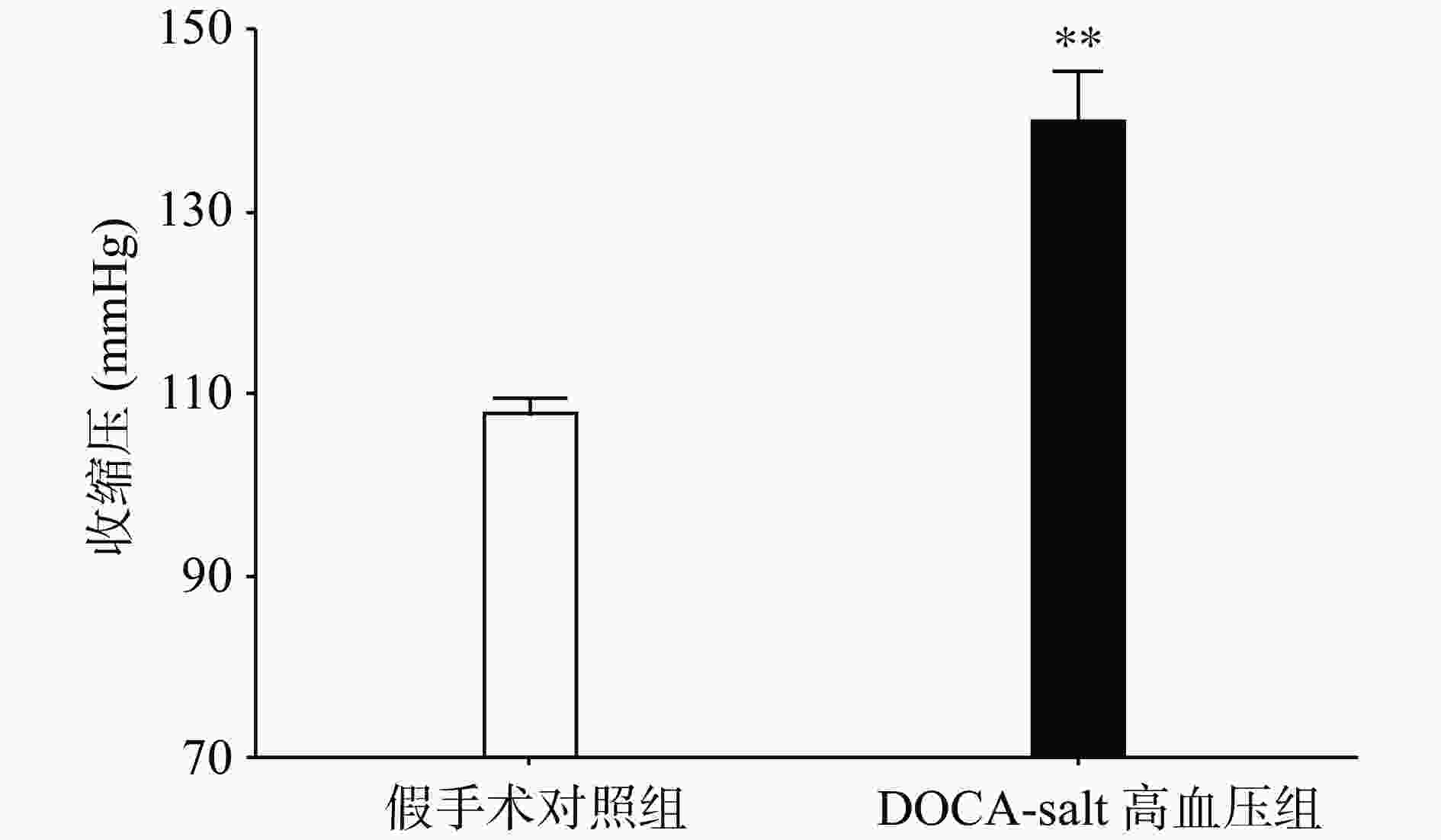
手术后分别测量高血压模型组和假手术组动物的血压水平,发现与假手术组相比,高血压模型组动物的收缩压水平显著上升(P<0.01,图1)。
-
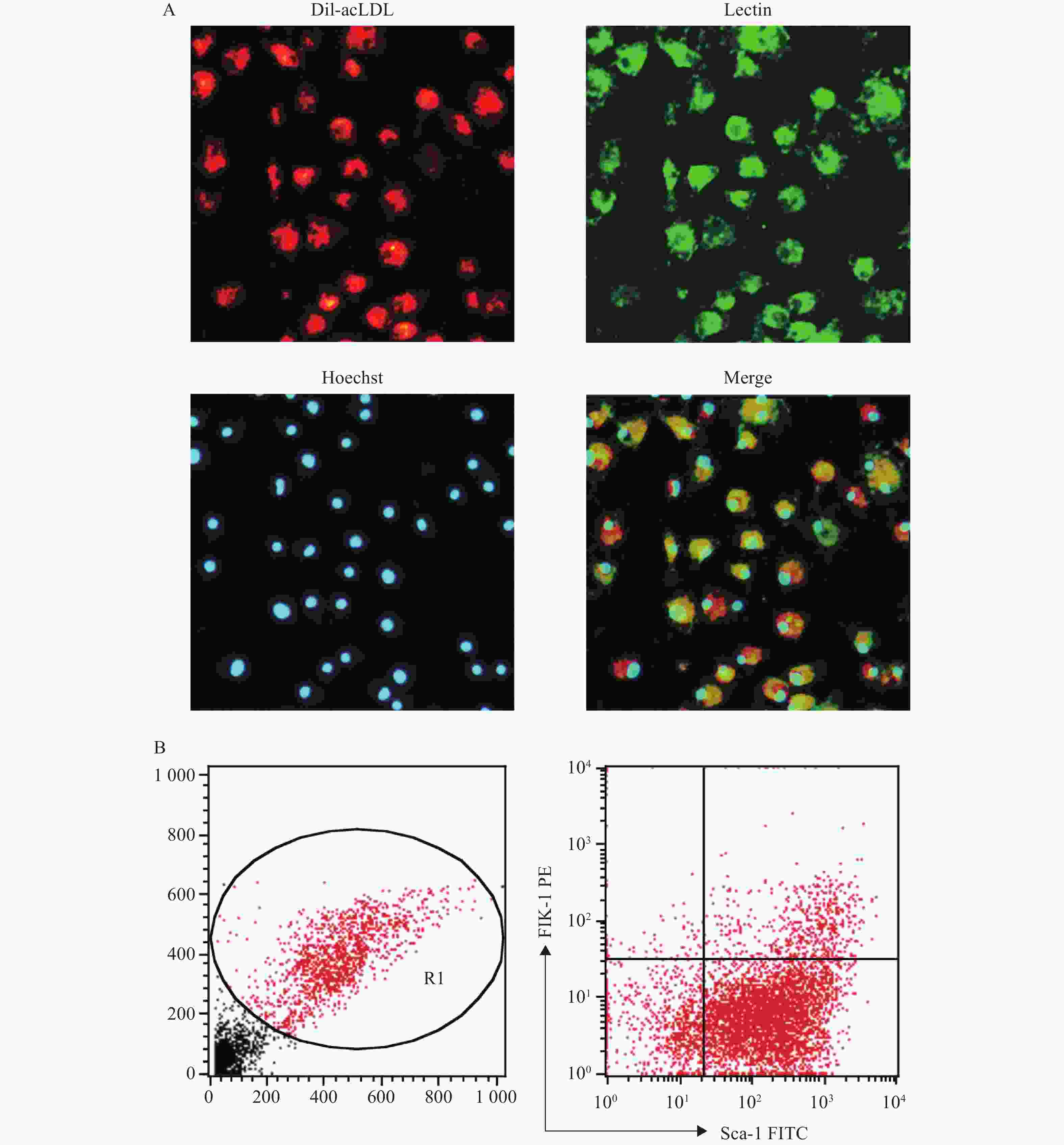
将培养至第7天的骨髓来源的EPC通过荧光显微镜法鉴定,显示Dil-acLDL/Lectin/Hoechst 33258三染阳性的细胞的比例为87.18%;通过流式细胞仪法鉴定,显示Sca-1和Flk-1双标阳性的细胞比例为(11.2±1.5):1,见图2。以上结果与文献报道中使用同类鉴定方法的结果相近[7-10]。
-
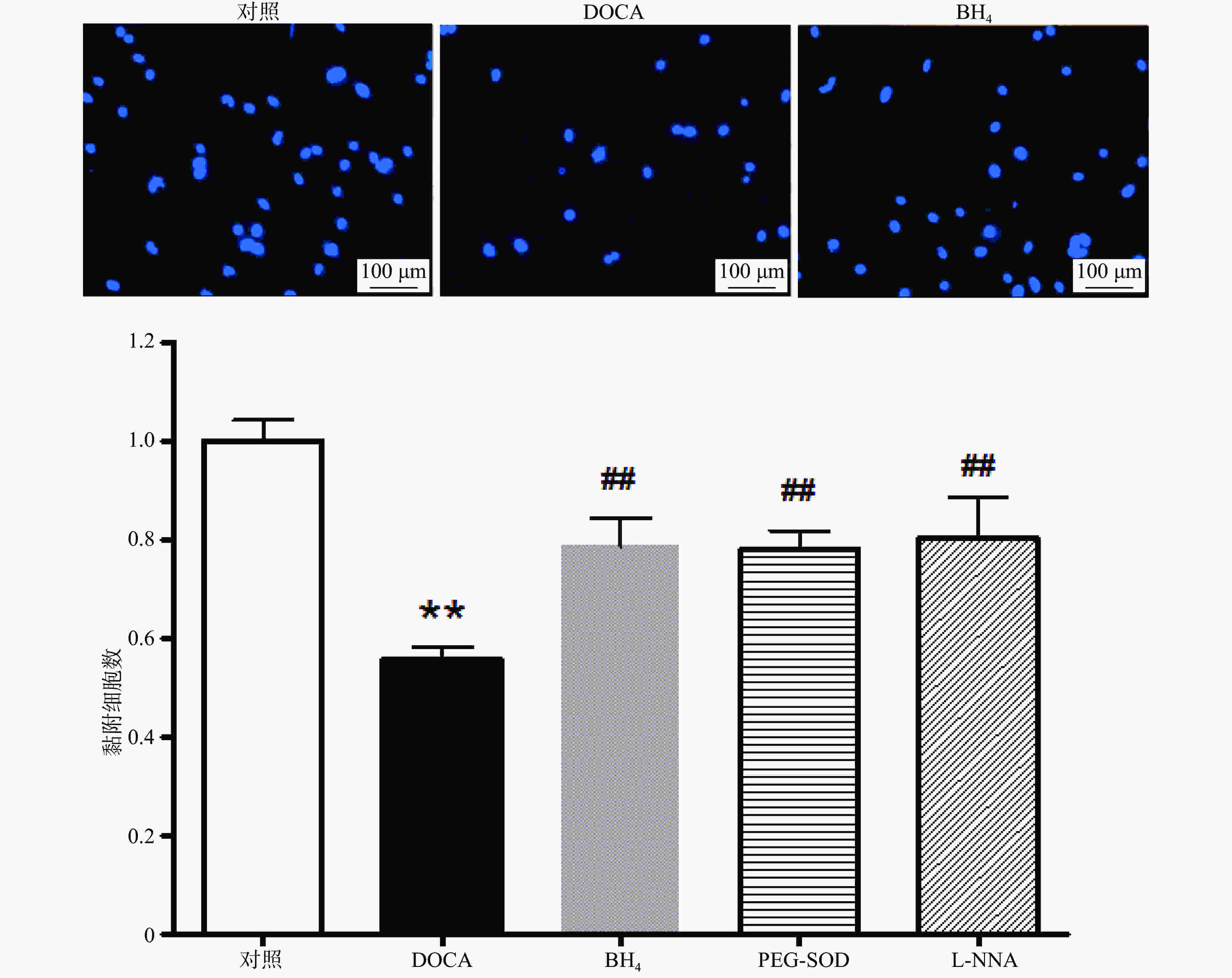
与对照组相比,DOCA-salt高血压小鼠EPC的黏附功能受损,而分别用BH4 (10 µmol/L), PEG-SOD (100 U/L),L-NNA (0.8 mmol/L)3种药物孵育24 h后,其黏附功能得到显著改善(图3)。
-
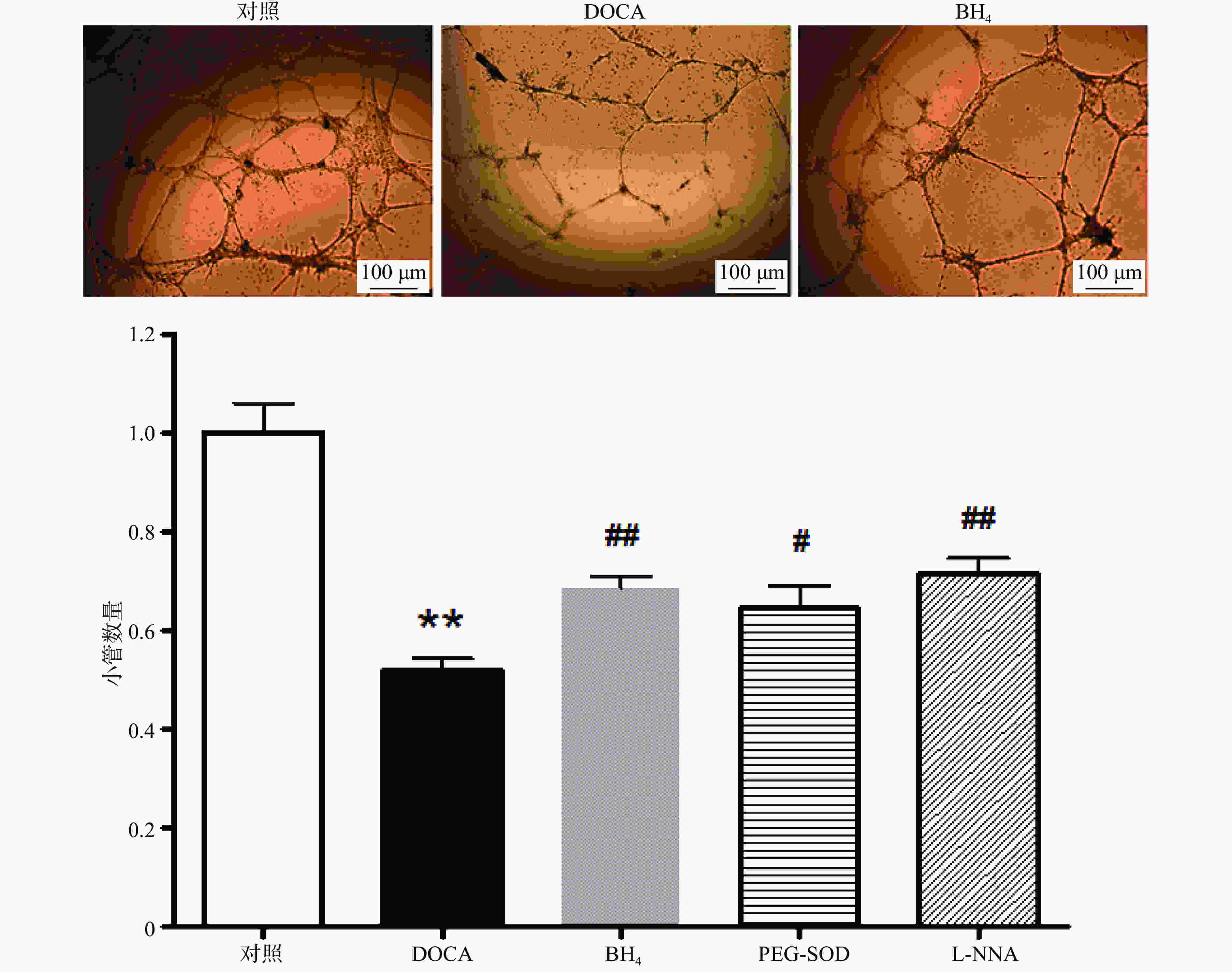
与对照组相比,DOCA-salt高血压小鼠EPC的小管形成功能受损,而分别用BH4 (10 µmol/L), PEG-SOD (100 U/L),L-NNA (0.8 mmol/L)3种药物孵育24 h后,其小管形成功能得到显著改善(图4)。
-
本研究发现,BH4、PEG-SOD和L-NNA均可有效改善DOCA-salt高血压小鼠EPC受损的功能。但此3种药物改善DOCA-salt小鼠EPC功能的具体机制尚有待进一步探究。
近年来研究发现,eNOS除了合成一氧化氮(nitric oxide, NO)外,在某些病理情况下,还是超氧阴离子(O2-)的重要来源。BH4是eNOS的必要辅助因子,影响NO和O2-的生成。BH4充足时eNOS催化底物L-精氨酸和O2-生成L-胍氨酸和NO;BH4缺乏时,eNOS则发生脱偶联,主要催化O2-产生[5-6, 11]。BH4缺乏是高血压、糖尿病等疾病中内皮功能异常的重要原因[11]。
本课题组和他人曾利用DOCA-salt高血压动物进行过大量血管生物学研究[4-6, 12],发现该动物血管功能异常与血管组织eNOS脱偶联有关。另外首次发现,DOCA-salt高血压小鼠EPC中BH4水平显著下降,出现eNOS脱偶联,而且eNOS脱偶联所引起的O2-水平升高是导致EPC功能异常的重要机制之一[5]。接着,我们还尝试采用一些药物手段对eNOS脱偶联相关的信号通路/分子进行了干预:①通过外源性补充BH4促进EPC的eNOS复偶联;②用L-NNA抑制脱偶联的eNOS活性,使其产生的O2-水平降低;③补充PEG-SOD直接清除EPC中升高的O2-[5]。我们发现,这些药物干预手段都能显著降低DOCA-salt高血压小鼠EPC的O2-水平[5]。本研究在这些发现的基础上进一步证实,这3种药物干预手段也能显著改善DOCA-salt高血压小鼠EPC的黏附功能和小管形成功能。这些结果也反过来验证了DOCA-salt小鼠EPC功能异常与eNOS脱偶联的关系。
通过本研究证实,BH4、PEG-SOD、L-NNA均可有效改善DOCA-salt高血压小鼠EPC受损的功能。这些研究结果可为高血压血管并发症的治疗提供参考,也可为改善高血压EPC移植治疗效果提供实验基础。
Improvement of impaired endothelial progenitor cell functions by in vitro drug interference in DOCA-salt hypertensive mice
doi: 10.12206/j.issn.1006-0111.201912074
- Received Date: 2019-12-16
- Rev Recd Date: 2020-03-05
- Available Online: 2020-05-20
- Publish Date: 2020-05-01
-
Key words:
- endothelial progenitor cells /
- hypertension /
- tetrahydrobiopterin /
- superoxide dismutase /
- N(G)-nitro-L-arginine
Abstract:
| Citation: | SHANG Liuwenxin, SUN Xin, PENG Cheng, XIE Hehui, CHEN Fengyuan, ZHANG Chuan. Improvement of impaired endothelial progenitor cell functions by in vitro drug interference in DOCA-salt hypertensive mice[J]. Journal of Pharmaceutical Practice and Service, 2020, 38(3): 221-226. doi: 10.12206/j.issn.1006-0111.201912074 |







 DownLoad:
DownLoad: